Figures & data
Figure 1 (A) Synthetic route of DSPE-PEG-Imine-MTX conjugate via imine reaction between the aldehyde group of DSPE-PEG-CHO and the aromatic amino group of MTX. (B) Schematic representation of preparation of MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR (M-CUR), DSPE-PEG-Amide-MTX nanoparticles (MTX-Amide-M-CUR), and DSPE-PEG-Imine-MTX nanoparticles (MTX-Imine-M-CUR). (C) Schematic representation of active selective cellular uptake via folate receptor-mediated endocytosis, pH-controlled intracellular dual-drug release, and combination therapy of MTX-Imine-M-CUR nanoparticles after passive tumor accumulation by EPR effect.
Abbreviations: AcOH, acetic acid; CHO, aldehyde group; CUR, curcumin; DHFR, dihydrofolate reductase; DMSO, dimethyl sulfoxide; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; EPR, enhanced permeability and retention; MTX, methotrexate.
![Figure 1 (A) Synthetic route of DSPE-PEG-Imine-MTX conjugate via imine reaction between the aldehyde group of DSPE-PEG-CHO and the aromatic amino group of MTX. (B) Schematic representation of preparation of MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR (M-CUR), DSPE-PEG-Amide-MTX nanoparticles (MTX-Amide-M-CUR), and DSPE-PEG-Imine-MTX nanoparticles (MTX-Imine-M-CUR). (C) Schematic representation of active selective cellular uptake via folate receptor-mediated endocytosis, pH-controlled intracellular dual-drug release, and combination therapy of MTX-Imine-M-CUR nanoparticles after passive tumor accumulation by EPR effect.Abbreviations: AcOH, acetic acid; CHO, aldehyde group; CUR, curcumin; DHFR, dihydrofolate reductase; DMSO, dimethyl sulfoxide; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; EPR, enhanced permeability and retention; MTX, methotrexate.](/cms/asset/94fbc829-c724-495c-b1bb-1b6894f9dbab/dijn_a_12193914_f0001_c.jpg)
Figure 2 Hydrodynamic size distribution, zeta potential, and TEM images of (A) M-CUR, (B) MTX-Amide-M-CUR, and (C) MTX-Imine-M-CUR. (D) XRD spectra of CUR, MTX-Amide-M nanocarriers, MTX-Amide-M nanocarriers/CUR mixture, MTX-Amide-M-CUR nanosystems, MTX-Imine-M nanocarriers, MTX-Imine-M nanocarriers/CUR mixture, and MTX-Imine-M-CUR nanosystems. (E) In vitro release profiles of CUR from M-CUR, MTX-Amide-M-CUR, and MTX-Imine-M-CUR in PBS buffer (pH 7.4 and 5.0) at 37°C. (F) In vitro release profiles of MTX from MTX-Amide-M-CUR and MTX-Imine-M-CUR in PBS buffer (pH 7.4 and 5.0) at 37°C.
Abbreviations: CUR, curcumin; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate; PBS, phosphate buffer saline; TEM, transmission electron microscope; XRD, X-ray diffractometer.
![Figure 2 Hydrodynamic size distribution, zeta potential, and TEM images of (A) M-CUR, (B) MTX-Amide-M-CUR, and (C) MTX-Imine-M-CUR. (D) XRD spectra of CUR, MTX-Amide-M nanocarriers, MTX-Amide-M nanocarriers/CUR mixture, MTX-Amide-M-CUR nanosystems, MTX-Imine-M nanocarriers, MTX-Imine-M nanocarriers/CUR mixture, and MTX-Imine-M-CUR nanosystems. (E) In vitro release profiles of CUR from M-CUR, MTX-Amide-M-CUR, and MTX-Imine-M-CUR in PBS buffer (pH 7.4 and 5.0) at 37°C. (F) In vitro release profiles of MTX from MTX-Amide-M-CUR and MTX-Imine-M-CUR in PBS buffer (pH 7.4 and 5.0) at 37°C.Abbreviations: CUR, curcumin; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate; PBS, phosphate buffer saline; TEM, transmission electron microscope; XRD, X-ray diffractometer.](/cms/asset/c441cbf9-ca15-44b4-8137-d887361429ad/dijn_a_12193914_f0002_c.jpg)
Figure 3 CLSM images of folate receptor-overexpressing (A) HeLa and (B) MCF-7 cells incubated with free CUR, M-CUR, and MTX-Imine-M-CUR for 0.5 and 2 h.
Notes: DAPI (false-color blue) was used to identify the nucleus. Scale bars are 15 µm.
Abbreviations: CLSM, confocal laser scanning microscopy; CUR, curcumin; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate.
![Figure 3 CLSM images of folate receptor-overexpressing (A) HeLa and (B) MCF-7 cells incubated with free CUR, M-CUR, and MTX-Imine-M-CUR for 0.5 and 2 h.Notes: DAPI (false-color blue) was used to identify the nucleus. Scale bars are 15 µm.Abbreviations: CLSM, confocal laser scanning microscopy; CUR, curcumin; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate.](/cms/asset/9b7734da-7248-4947-9474-e98424d3afc7/dijn_a_12193914_f0003_c.jpg)
Figure 4 (A) CLSM images, (B) flow cytometry profiles, and (C) mean fluorescence intensity of HeLa cells incubated with free CUR, M-CUR, MTX-Amide-M-CUR (without/with FA pretreatment), or MTX-Imine-M-CUR (without/with FA pretreatment) for 2 h. Error bars indicate SD (n=4). *P<0.05. (D) Subcellular location of MTX-Imine-M-CUR in HeLa cells after incubation for 1 h.
Notes: DAPI (false-color blue) and LysoTracker Red (false-color red) were used to identify the nucleus and lysosomes, respectively. Scale bars are 20 µm.
Abbreviations: CLSM, confocal laser scanning microscopy; CUR, curcumin; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; FA, folic acid; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate; NA, no answer.
![Figure 4 (A) CLSM images, (B) flow cytometry profiles, and (C) mean fluorescence intensity of HeLa cells incubated with free CUR, M-CUR, MTX-Amide-M-CUR (without/with FA pretreatment), or MTX-Imine-M-CUR (without/with FA pretreatment) for 2 h. Error bars indicate SD (n=4). *P<0.05. (D) Subcellular location of MTX-Imine-M-CUR in HeLa cells after incubation for 1 h.Notes: DAPI (false-color blue) and LysoTracker Red (false-color red) were used to identify the nucleus and lysosomes, respectively. Scale bars are 20 µm.Abbreviations: CLSM, confocal laser scanning microscopy; CUR, curcumin; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; FA, folic acid; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate; NA, no answer.](/cms/asset/ca8e1faf-3078-4603-be58-7863cac42f21/dijn_a_12193914_f0004_c.jpg)
Figure 5 (A) Intracellular drug/nanocarriers distribution of HeLa cells treated with MTX-Imine-M-CUR for 1 and 6 h. DSPE-PEG-Cy5.5 (false-color red) was used to label nanocarriers. (B, C) In vitro cell viability of (B) HeLa cells and (C) MCF-7 cells incubated with free CUR, free CUR/MTX mixture, M-CUR nanosystems, MTX-Amide-M-CUR nanosystems, or MTX-Imine-M-CUR nanosystems for 24 h. (D, E) In vitro cell viability of (D) HeLa and (E) MCF-7 cells incubated with MTX-Imine-M-CUR nanosystems without/with FA pretreatment for 24 h.
Note: Error bars indicate SD (n=4).
Abbreviations: C, cytoplasm; CUR, curcumin; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; FA, folic acid; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate; N, nucleus.
![Figure 5 (A) Intracellular drug/nanocarriers distribution of HeLa cells treated with MTX-Imine-M-CUR for 1 and 6 h. DSPE-PEG-Cy5.5 (false-color red) was used to label nanocarriers. (B, C) In vitro cell viability of (B) HeLa cells and (C) MCF-7 cells incubated with free CUR, free CUR/MTX mixture, M-CUR nanosystems, MTX-Amide-M-CUR nanosystems, or MTX-Imine-M-CUR nanosystems for 24 h. (D, E) In vitro cell viability of (D) HeLa and (E) MCF-7 cells incubated with MTX-Imine-M-CUR nanosystems without/with FA pretreatment for 24 h.Note: Error bars indicate SD (n=4).Abbreviations: C, cytoplasm; CUR, curcumin; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; FA, folic acid; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate; N, nucleus.](/cms/asset/028074bd-1035-4067-bad1-2abaef8fc757/dijn_a_12193914_f0005_c.jpg)
Figure 6 In vivo antitumor efficacy of HeLa tumor-bearing nude mice after intravenous injection of 0.9% NaCl, free CUR, free CUR/MTX, M-CUR, MTX-Amide-M-CUR, or MTX-Imine-M-CUR at an equivalent dose of CUR (8 mg/kg).
Notes: (A) Tumor volumes changes, (B) body weight changes, (C) isolated tumor weight, and (D) representative H&E staining histologic images from the tumor tissues. Error bars indicate SD (n=5). *P<0.05.
Abbreviations: CUR, curcumin; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate.
![Figure 6 In vivo antitumor efficacy of HeLa tumor-bearing nude mice after intravenous injection of 0.9% NaCl, free CUR, free CUR/MTX, M-CUR, MTX-Amide-M-CUR, or MTX-Imine-M-CUR at an equivalent dose of CUR (8 mg/kg).Notes: (A) Tumor volumes changes, (B) body weight changes, (C) isolated tumor weight, and (D) representative H&E staining histologic images from the tumor tissues. Error bars indicate SD (n=5). *P<0.05.Abbreviations: CUR, curcumin; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate.](/cms/asset/b27b48e0-8fbc-4900-b0b1-ae34413e4f96/dijn_a_12193914_f0006_c.jpg)
Figure S1 (A) 1H NMR, (B) UV–vis absorption, (C) XRD, (D) MALDI-TOF-MS, and (E) GPC spectra of DSPE-PEG-CHO, MTX, and DSPE-PEG-Imine-MTX amphiphilic polymer prodrug.
Abbreviations: 1HNMR, 1H nuclear magnetic resonance; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; DSPE-PEG-CHO, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(aldehyde[polyethylene glycol]-2000); GPC, gel permeation chromatography; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MTX, methotrexate; UV–vis, ultraviolet–visible; XRD, X-ray diffractometer.
![Figure S1 (A) 1H NMR, (B) UV–vis absorption, (C) XRD, (D) MALDI-TOF-MS, and (E) GPC spectra of DSPE-PEG-CHO, MTX, and DSPE-PEG-Imine-MTX amphiphilic polymer prodrug.Abbreviations: 1HNMR, 1H nuclear magnetic resonance; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; DSPE-PEG-CHO, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(aldehyde[polyethylene glycol]-2000); GPC, gel permeation chromatography; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MTX, methotrexate; UV–vis, ultraviolet–visible; XRD, X-ray diffractometer.](/cms/asset/b5eeea8d-a2cf-406b-abd2-16a6df181291/dijn_a_12193914_sf0001_c.jpg)
Figure S2 In vitro stability of hydrodynamic size distribution of MTX-Imine-M-CUR nanosystems in (A) water and (B) PBS over 72 h. Error bars indicate SD (n=3).
Abbreviations: CUR, curcumin; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate; PBS, phosphate buffer saline; PDI, polydispersity index.
![Figure S2 In vitro stability of hydrodynamic size distribution of MTX-Imine-M-CUR nanosystems in (A) water and (B) PBS over 72 h. Error bars indicate SD (n=3).Abbreviations: CUR, curcumin; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-2000]; M-CUR, MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR; MTX, methotrexate; PBS, phosphate buffer saline; PDI, polydispersity index.](/cms/asset/8f454de9-e127-4fdb-873c-b628672acaf5/dijn_a_12193914_sf0002_c.jpg)
Figure S3 Blood circulation of MTX-Imine-M-CUR nanosystems. Concentration of CUR in blood at different time points after intravenous injection of MTX-Imine-M-CUR nanosystems.
Notes: The free CUR was used as a control. Error bars indicate SD (n=3).
Abbreviations: CUR, curcumin; MTX, methotrexate.
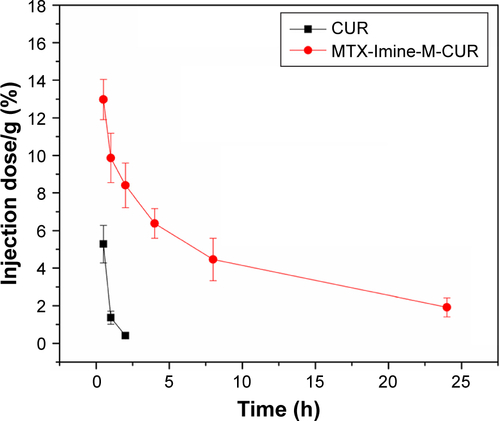
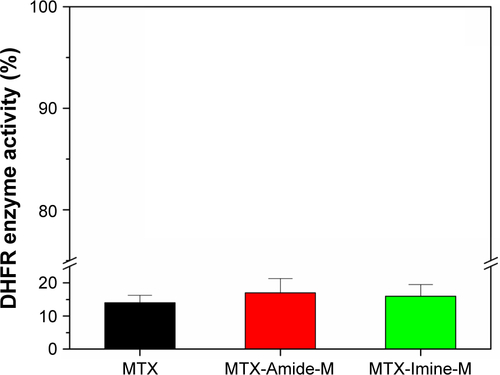
Figure S4 Relative DHFR activity in free MTX, MTX-Amide-M, and MTX-Imine-M at a MTX concentration of 0.1 µg/mL (very low concentration).
Notes: Error bars indicate SD (n=3). The enzymatic activity of DHFR in the presence of MTX or MTX conjugates was determined by a DHFR assay kit (Sigma-Aldrich) based on the NADPH-dependent reduction of dihydrofolic acid to tetrahydrofolic acid. The assay was conducted according to the reported literature.Citation1 Free MTX, MTX-Amide-M, and MTX-Imine-M showed a comparable inhibition effect of DHFR enzyme activity.
Abbreviations: DHFR, dihydrofolate reductase; MTX, methotrexate; NADPH, nicotinamide adenine dinucleotide phosphate.