Figures & data
Table 1 Signals and signaling molecules released during apoptosis and phagocytosis
Figure 1 The role of neutrophils in the etiopathogenesis of SLE.
Notes: Activated neutrophils release NETs covered with α-NE, α-LF, α-MPO, or α-LL-37. Chromatin and associated compounds are hallmark antigens of the autoimmune response of patients with SLE. Decreased activity of DNase I combined with a general (anti-inflammatory) clearance deficiency leads to the accumulation of NETs covered with proinflammatory and cytotoxic intracellular constituents. Opsonization of NETs with autoantbodies comes with immune complex formation followed by inflammatory clearance by blood-borne phagocytes. This process causes inflammation and tissue damage, thus stimulating pDC to secrete IFN-α and IL-6, ultimately resulting in the so-called “IFNα signature” typical of SLE. The pro-inflammatory cytokines secreted by pDCs induce long-lived plasma cell formation and massive autoantibody production.
Abbreviations: α-NE, antibodies against neutrophil elastase; α-MPO, antibodies against myeloperoxidase; α-LF, antibodies against lactoferrin; NET, neutrophil extracellular trap; IFN-α, interferon-alpha; IL-6, interleukin-6; pDC, plasmacytoid dendritic cells; SLE, systemic lupus erythematosus; NETosis, neutrophil extracellular trap formation; NETs, neutrophil extracellular traps; MΦ: macrophage.
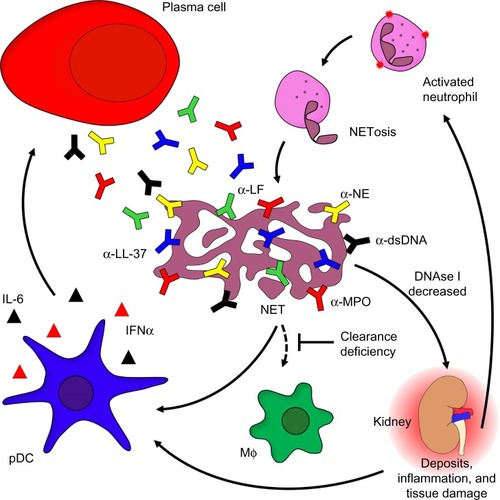
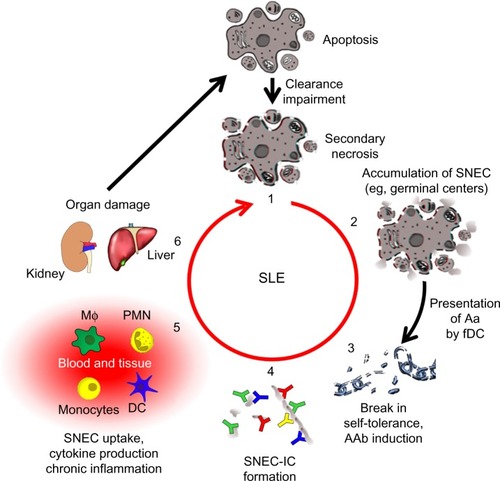
Figure 2 The vicious cycle of SLE.
Notes: A deficiency in the clearance of apoptotic cells leads to autoimmunity and chronic inflammation (1). when apoptotic cells fail to be cleared in time, they get secondary necrosis, leading to the accumulation of SNEC (2). Self-tolerance is broken when SNEC-derived autoantigens (Aag) are presented to autoreactive B cells by fDC. with help from autoreactive helper T cells, these B cells undergo affinity maturation and differentiate into memory B cells, thus establishing autoimmunity (3). IC are formed when autoantibodies (AAb) encounter SNEC in circulation or tissue (4). Newly formed SNEC-IC are then processed by blood-borne phagocytes and dendritic cells (DC) accompanied by the secretion of pro-inflammatory cytokines (5). This in turn leads to severe organ damage and cell death fueling the vicious cycle that maintains chronic inflammation (6).
Abbreviations: SNEC, secondary necrotic cell-derived material; fDC, follicular dendritic cells; IC, immune complex; SLE, systemic lupus erythematosus; DC, dendritic cells; MΦ: macrophage, PMN: polymorphonuclear leukocytes.