Figures & data
Figure 1 Example of the application of the investigational medical product (Healy device); 1: Adhesive electrodes, 2: Cables, 3: Healy device.
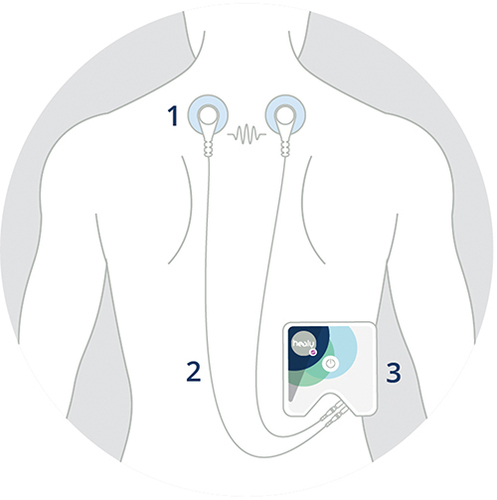
Table 1 Summary of Demographic Data, All Study Participants and Participants Suffering from the Different Indications, Mean Values and Standard Deviation in Brackets. Second Line Minimum and Maximum
Table 2 Results of Repeated Measurement ANOVA and Friedman Test of SF-36 Scores and Physical and Mental Components. Clinically Significant Results (P < 0.05) are Indicated in Bold
Figure 2 Temporal changes in quality of life as assessed by the patients; x-axis study day, y-axis SF-36 score; Mean values and 95% confidence interval in SF-36 total scores (a), physical component score (b) and mental component score (c).
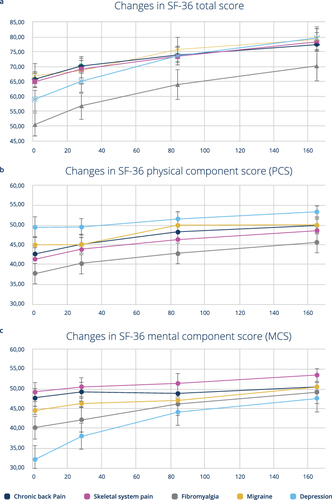
Figure 3 Changes in secondary endpoint variables during the study: Pain Assessment Score for chronic back pain (a) and skeletal system pain group (b); Mental Illness Assessment (PHQ 9), Anxiety assessment (GAD-7), Sleep quality (ISI) valid for indications fibromyalgia (c) and depression (d) only; MiDAS in the migraine group (e) only; Mean values and 95% confidence interval.
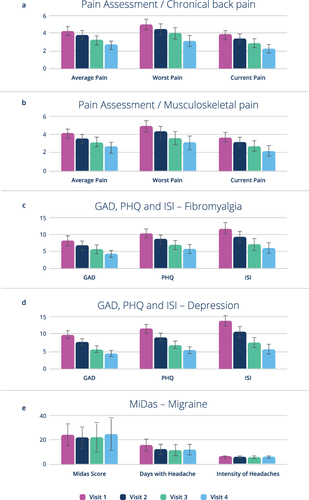
Table 3 Results of Repeated Measurement ANOVA and Friedman Test of Secondary Endpoints, Pain Assessment Score for the Chronic Back Pain and Skeletal System Pain Group, Generalized Anxiety Disorder (GAD-7), Patient Health Questionnaire for the Assessment of Depression Severity (PHQ-9) and Insomnia Severity Index (ISI) for Fibromyalgia- and Depression Groups and Migraine Disability Assessment Score (MiDAS) for the Migraine Group. Clinically Significant Results (P < 0.05) are Indicated in Bold
Table 4 Adverse Events According to ICD-10 Class Assignment in Descending Order According to the Frequencies
Table 5 Serious Adverse Events per Patient
Table 6 Minimum Clinically Important Differences as Mentioned in Scientific Publications
