Figures & data
Table 1 The characteristics of included studies
Figure 1 Study selection procedure with PRISMA flow diagram.
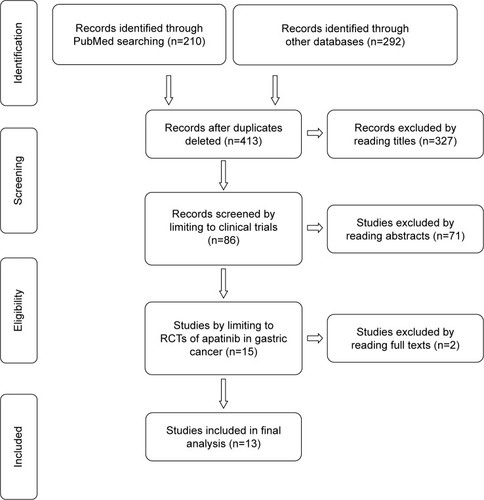
Figure 2 Forest plot of ORR (A) and DCR (B) between apatinib-alone or apatinib-based regimens and no-apatinib groups.
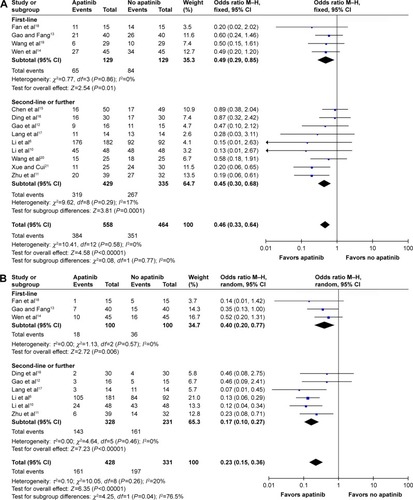
Figure 3 Forest plot of hematological toxicity (A), digestive events (B), and general events (C1 and C2) between apatinib-alone or apatinib-based regimens and no-apatinib groups.
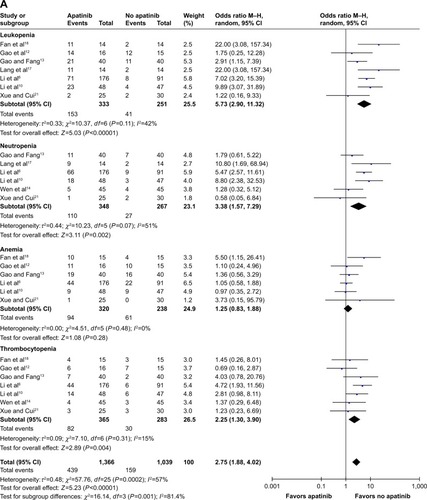
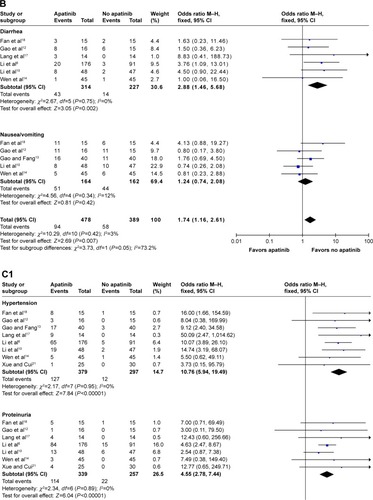
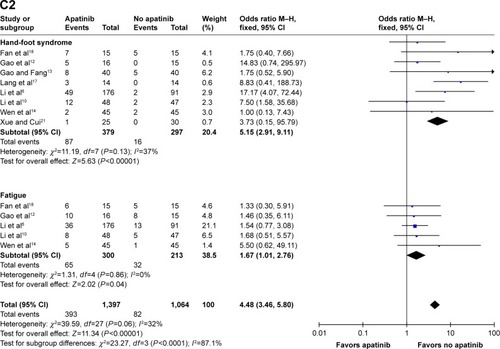
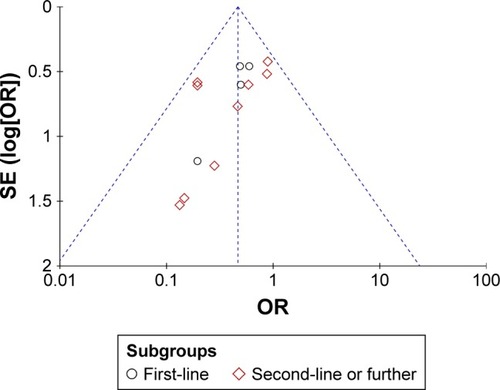
Figure 4 Funnel plot for publication bias with ORR.