Figures & data
Table I. Five hypofractionated dose schedules.
Figure 1. (A) A set of 3-mm-thick contrast computed tomography images was acquired for treatment planning. Three gold markers were inserted into the prostate. One of the three gold markers is not seen because it was inserted into a different axis. A balloon was inserted into the rectum and filled with 100 ml of saline. (B) Treatment planning was performed using bilateral beams with the Eclipse proton beam planning system (Ver. 8.1; Varian Medical System, Palo Alto, CA, USA). The planning goals included that at least 95% of the PTV received the prescribed dose, and that the volume receiving 50 Gy EQD2 should not exceed 30% for the rectum and bladder.
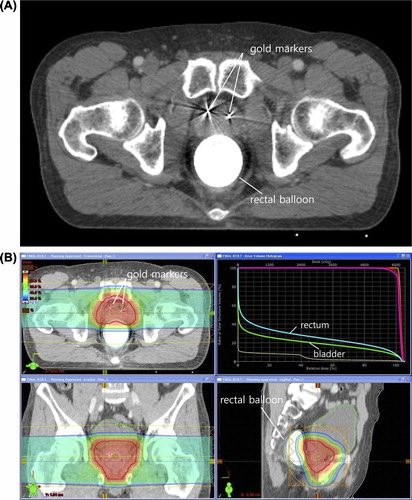
Table II. Patient characteristics.
Table III. Maximum acute and late toxicity.
Table IV. Patient-reported measures.
Figure 2. Biochemical failure free survival (BCFFS) curves, according to the American Society for Therapeutic Radiology and Oncology (ASTRO) and Nadir + 2 ng/ml definitions, are presented. The four-year ASTRO and Nadir + 2 ng/ml BCFFS rates were 85% and 86%, respectively.
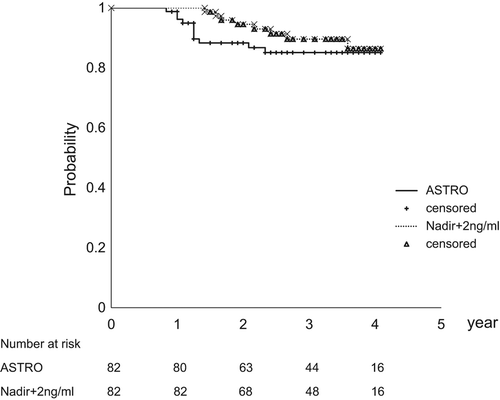
Table V. Previous studies.