Figures & data
Table 1. Summary of global regulatory authorities and PPP testing requirements.
Figure 1. The mouse palatability study was conducted in a stepwise manner using a minimum number of animals and also minimizing excessively high-dose levels.
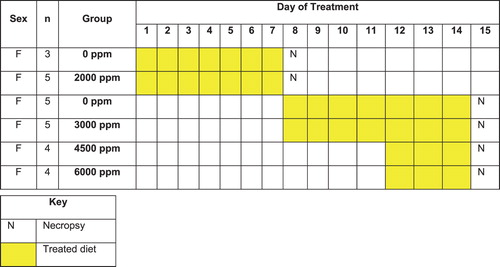
Figure 2. The dog palatability study was conducted in a stepwise manner using a minimum number of animals, with no separate control group but the individual animals acting as their own controls by using pre-exposure food consumption values. Severe tolerability issues were encountered with administration of the test material to dogs, hence the need to assess dietary (ad libitum, restricted, flavored, and tinned feed), capsule and gavage dosing as the route of administration.
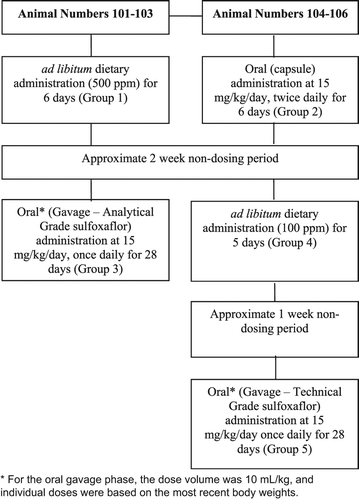
Table 2. Animal assignment for the probe part of the 28-day dog study.
Figure 3. The 90-day rat study design for sulfoxaflor was enhanced with a number of ‘add-ons’ including endpoints for toxicokinetics (indicated in red text), neurotoxicity (pink text), and immunotoxicity (blue-colored text). toxicokinetics, TK; sheep red blood cell, SRBC; functional observational battery, FOB; plaque-forming cell, PFC.
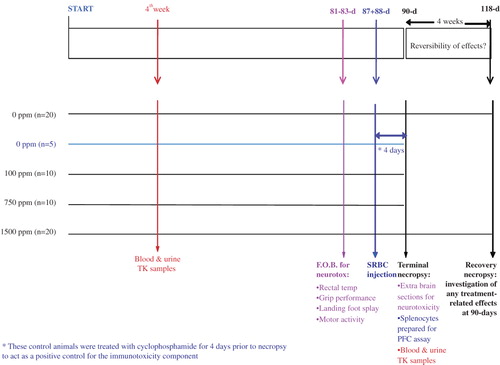
Table 3. Comparison of the diurnal systemic dose of sulfoxaflor to rabbits administered via bolus oral gavage with that via the dietary route.
Table 4. Toxicokinetic profile of sulfoxaflor in rats.
Table 5. Summary of the excretion of the bolus oral dose used for the estimation of the absorption of sulfoxaflor in mice.
Table 6. Comparative toxicokinetics of sulfoxaflor across doses and species.
Table 7. Toxicokinetics of sulfoxaflor across doses, species and life stages.
Table 8. Comparison of the expected and the observed liver effects in 28- and 90-day mouse studies.
Table 9. Sulfoxaflor: temporal and dose response and reversibility for increased relative liver weights rats.
