Figures & data
FIGURE 1. Clinical features of the family affected with ARS. (A) Symbols for affected individuals are colored in. The proband (III4) has congenital glaucoma and accompanying congenital heart disease due to PDA. His father (II3) only has glaucoma. His sister (III3) who died in 2010 due to dilated cardiomyopathy, heart failure, cardiogenic shock and accompanying pulmonary edema. (B) Sequence chromatogram indicates a G to T transition of nucleotide 127 in the proband (III4) and his father (II3). (C) The proband’s father has a congenital detachment of the retina as well as glaucoma. (D) The proband also suffers from glaucoma but neither of them have other facial anomalies.
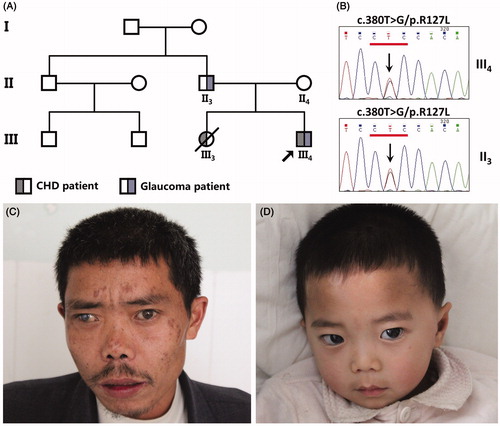
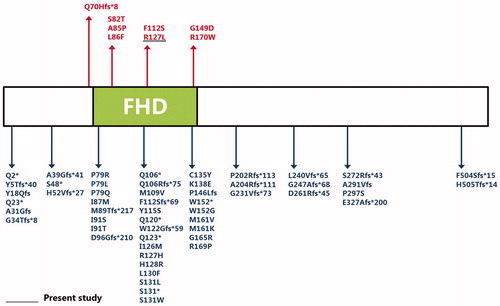
TABLE 1. Summary of identified FOXC1 defects with cardiac anomalies in Axenfeld–Rieger syndrome.
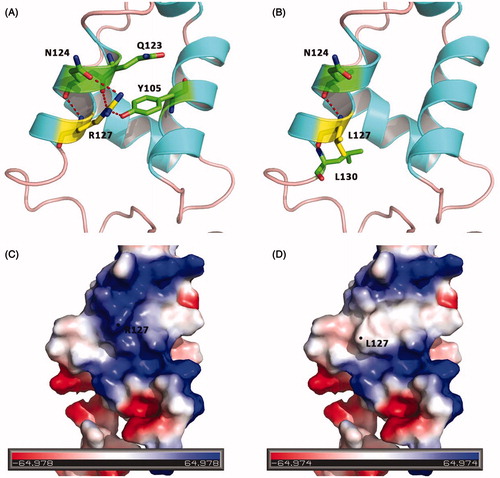
FIGURE 2. Molecular models of the FOXC1 R127L mutation. (A, B) Diagrams displaying the position of Arg127 and Leu127 (both in yellow), respectively. Red dashed lines show hydrogen bonds. (A) Arg127 linked with Tyr105, Gln123 and Asn124 (both in green) by hydrogen bonds. But (B) Leu127 can only link with Asn124 and Leu130 (both in green) by a hydrogen bond. (C, D) Surface electrostatic charge distribution of FOXC1. As the color legend indicates, the red color (negative potential) arises from an excess of negative charges near the surface and the blue color (positive potential) occurs when the surface is positively charged. Obviously, the alteration of Arg to Leu in 127 reduces the positive potential. Molecular diagrams were drawn with PyMOL (http://www.pymol.org).