Figures & data
Figure 1. Structures of ICL670 (I) and of our calix[4]arene derivatives (II–IV) previously described as antiproliferative compounds.
![Figure 1. Structures of ICL670 (I) and of our calix[4]arene derivatives (II–IV) previously described as antiproliferative compounds.](/cms/asset/c23411c3-ae66-49a5-88e7-f0433c8f65a7/ienz_a_489898_f0001_b.gif)
Scheme 1. Synthesis of calix[4]arenes 1-5. Reagents: (i) wet K2CO3, N-3-Bromopropyl-N,N-bis(methoxycarbonylmethyl)amine 9, CH3CN, KI, reflux; (ii) anhydrous THF, LiAlH4, 0°C then 90°C; (iii) anhydrous K2CO3, N-3-Bromopropyl-N,N-bis(methoxycarbonylmethyl)amine 9, CH3CN, KI, reflux; (iv) 1) aqueous solution of NaOH 4M, MeOH; 2) HCl, H2O.
![Scheme 1. Synthesis of calix[4]arenes 1-5. Reagents: (i) wet K2CO3, N-3-Bromopropyl-N,N-bis(methoxycarbonylmethyl)amine 9, CH3CN, KI, reflux; (ii) anhydrous THF, LiAlH4, 0°C then 90°C; (iii) anhydrous K2CO3, N-3-Bromopropyl-N,N-bis(methoxycarbonylmethyl)amine 9, CH3CN, KI, reflux; (iv) 1) aqueous solution of NaOH 4M, MeOH; 2) HCl, H2O.](/cms/asset/6f71cfb1-e829-4cb3-8388-cce88bbaf32d/ienz_a_489898_f0003_b.gif)
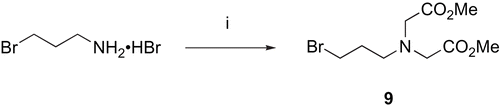
Scheme 2. Synthesis of N-3-Bromopropyl-N,N-bis(methoxycarbonylmethyl)amine 9. Reagents: (i) BrCH2COOMe, DIEA, MeCN, 0°C.
Scheme 3. Synthesis of calix[4]arenes 6–8. Reagents: (i) NaH, Br(CH2)5OC6H4CN, DMF; (ii) 1) LiN(TMS)2, THF; 2) HCl, EtOH; (iii) NH2OH.HCl, t-Bu-OK, DMSO.
![Scheme 3. Synthesis of calix[4]arenes 6–8. Reagents: (i) NaH, Br(CH2)5OC6H4CN, DMF; (ii) 1) LiN(TMS)2, THF; 2) HCl, EtOH; (iii) NH2OH.HCl, t-Bu-OK, DMSO.](/cms/asset/5fb5fdc3-9c0c-485f-800f-fac9e4ac88ff/ienz_a_489898_f0005_b.gif)
Figure 3. The ORTEP drawing of calix[4]arenes 1–3. Displacement ellipsoids are drawn at the 30% probability level, H atoms are omitted for clarity.
![Figure 3. The ORTEP drawing of calix[4]arenes 1–3. Displacement ellipsoids are drawn at the 30% probability level, H atoms are omitted for clarity.](/cms/asset/2fc48a00-a19f-4daf-9f49-3be0dcba16d0/ienz_a_489898_f0006_b.gif)
Table 1. 50% of the maximal dequenching of calcein (ED50).Comparison of iron(III) chelating efficiency by using calcein fluorescence measurements in a cell-free system. Fluorescence of 100 nM calcein (λExc = 485nm, λEm = 520nm) in Hepes buffer (20 mM HEPES, 150 mM NaCl, pH 7.3) was detected in a microplate fluorescence reader (free calcein). Iron(III) (1 µM) totally quenched the calcein fluorescence and addition of compounds including ICL670 used as a chelator reference led to a fluorescence recovery depending on the chelator concentration, the kinetic and stoichiometry of their iron binding affinity.
Table 2. Biological effect of a 72 h treatment of HepaRG cells with various calix[4]arene compounds. Cell viability was evaluated by cell nuclei counting after Hoechst stain. Parameters of the biphasic dose-response curves, including the IC50 values, were deduced from a four parameters fit (see methods). LDH leakage in cell supernatant for compound concentration 100µM was used as an index of membrane damages (cytotoxicity). Data expressed as a percent of the control (absence of compound) are the mean of three independent experiments.
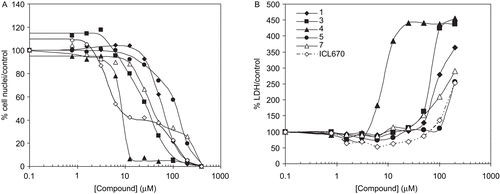
Figure 4. Effect of chelators on cell viability (A, cell nuclei counting after Hoechst stain) and cytotoxicity (B, LDH release in supernatant) in proliferating HepaRG cell cultures. Four days after seeding HepaRG cells were maintained in culture for 72 hours with various concentrations of compounds 1 (__♦__ ), 3 (__▪__), 4 (__▴__), 5 (__•__ ), 7 (__▵__) and ICL670 (−.-◊−.-)
![Figure 2. Structures of new calix[4]arene derivatives, 1–8.](/cms/asset/2761bf81-198a-4c75-bfa8-7f74397fdf04/ienz_a_489898_f0002_b.gif)