Figures & data
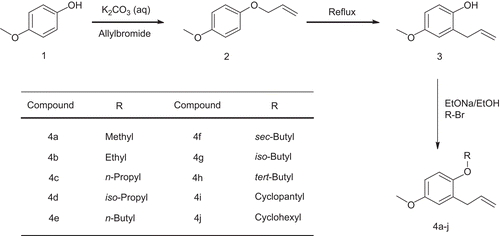
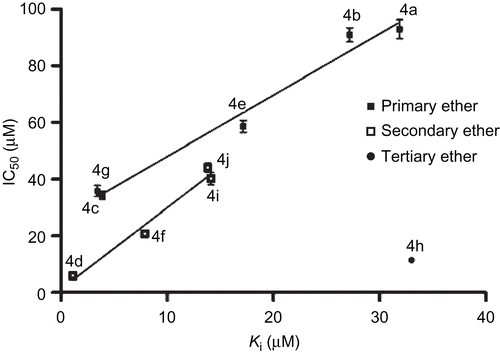
Table I. Enzyme inhibitory assessment and docking analysis data of consensus conformers of eugenol, methyl eugenol, methyl isoeugenol and compounds 4a–j. Ki (M): Estimate d Inhibition Constant. The IC50 (µM) values are given as mean ± SD.
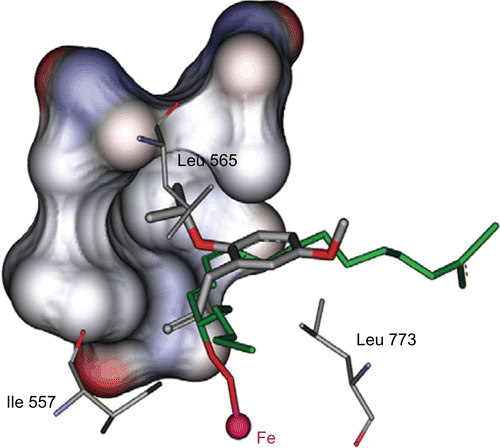
Figure 2. Stick (right) and solvent surface (left) view of flexible residues, surrounding the consensus bonding conformations of compounds 4a–j in the SLO active site.
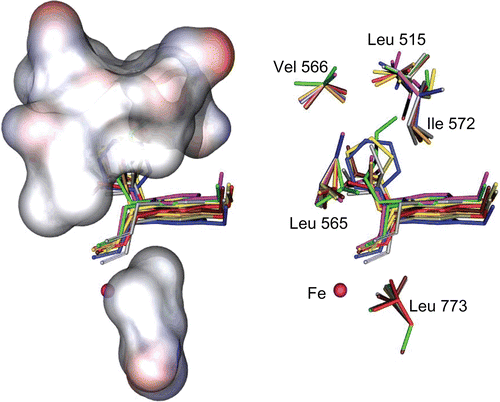
Figure 3. Multiple alignment of SLO (1ik3_A). The residues which have lipophilic interactions with docked ligands are highlighted in green respectively.

Figure 5. Stick view of the consensus bonding conformation of compound 4d which has lipophilic interaction through its isopropyl moiety with hydrophobic pockets (surface view) formed by Leu515, Trp519, Val566 and Ile572 side chain. In this figure we can see the orientation of linoleic acid (green stick) bonded to Fe via proxy bridge.