Figures & data
Figure 2. Reaction scheme for the reactivation of organophosphate-inhibited acetylcholinesterase (AChE) by oximes. The respective concentrations are: [EP] the phosphylated AChE, [OX] the reactivator, [EPOX] the Michaelis-type phosphyl-AChE-oxime-complex, [E] the active enzyme and [POX] the phosphylated oxime. KD is equal to the ratio [EP] × [OX]/[EPOX] and describes the dissociation constant which is inversely proportional to the affinity of the oxime to [EP], and kr is the rate constant for the displacement of the phosphyl residue from [EPOX], indicating the reactivity of the oxime.
![Figure 2. Reaction scheme for the reactivation of organophosphate-inhibited acetylcholinesterase (AChE) by oximes. The respective concentrations are: [EP] the phosphylated AChE, [OX] the reactivator, [EPOX] the Michaelis-type phosphyl-AChE-oxime-complex, [E] the active enzyme and [POX] the phosphylated oxime. KD is equal to the ratio [EP] × [OX]/[EPOX] and describes the dissociation constant which is inversely proportional to the affinity of the oxime to [EP], and kr is the rate constant for the displacement of the phosphyl residue from [EPOX], indicating the reactivity of the oxime.](/cms/asset/80fe925d-3936-4409-b68f-09d2803f96e5/ienz_a_504673_f0002_b.gif)
Table 1. Highest and lowest reactivation rate constants of oximes with OP-inhibited human AChE.
Table 2. Reactivation rate constants of selected oximes and OP.
Figure 3. Calculated acetylcholinesterase (AChE) activities of tabun- (A), cyclosarin- (B), paraoxon-ethyl- (C), and methamidophos-inhibited AChE (D) after reactivation by obidoxime (OBI, 10 µM), 2-PAM (100 µM), HI-6 (50 µM), and MMB-4 (100 µM). Based on experimental reactivation rate constantsCitation16, the AChE activities were calculated using the equation AChEt% = 100 × (1-exp−kobs × t).
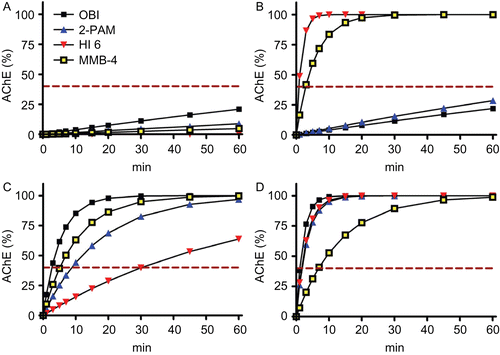
Figure 4. Double-logarithmic plot showing the relationship between reactivation half-time (t1/2, min) and oxime reactivity (kr in min−1). The t1/2 was calculated using equation (1) assuming five different dissociation constants (KD), i.e. 1 µM, 10 µM, 100 µM, 1000 µM, and 3000 µM for two different oxime concentrations, i.e. 10 µM (A) and 100 µM (B). The dashed line indicates a reactivation t1/2 of 5 min. In addition, reactivation half-times of selected oximes and organophosphorus compounds were included using measured reactivation rate constants (cf. ): Tabun and obidoxime (x), cyclosarin and pralidoxime (+), obidoxime (*), and HI-6 (▪).
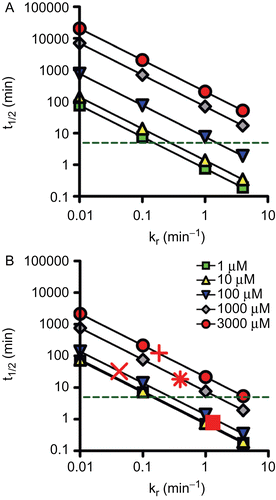
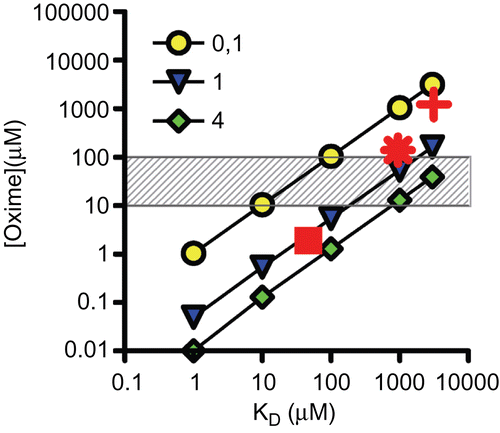
Figure 5. Double-logarithmic plot showing the relationship between oxime concentration (µM) and dissociation constant (KD in µM). Oxime concentrations necessary to obtain 40% reactivation of inhibited acetylcholinesterase within 10 min were calculated according toCitation17 for three different oxime reactivity constants (kr) of 0.1, 1 and 4 min−1. The hatched area resembles the range of clinically used oxime concentrations. In addition, necessary oxime concentrations of selected oximes and organophosphorus compounds were included using measured reactivation rate constants (cf. ): Cyclosarin and pralidoxime (+), obidoxime (*), and HI-6 (▪).