Figures & data
Table 1 Inhibition of acetylcholinesterase from Electrophorus electricus (EeAChE), human acetylcholinesterase (hAChE) and human butyrylcholinesterase (hBChE) by compounds 7–14.
Figure 2. Relevant nuclear overhauser effect enhancement of compound 12. The spectrum was recorded in CDCl3.
Scheme 1. Synthesis of compounds 7–10. Reagents and conditions: (a) PhNCS, ethanol 80°C, 7h; (a) 1. 1N NaOH, EtOH, 2. MeI, room temperature; (c) ethylenediamine or 1,3-diaminopropane, 2-methoxyethanol, 150°C, 24 h.
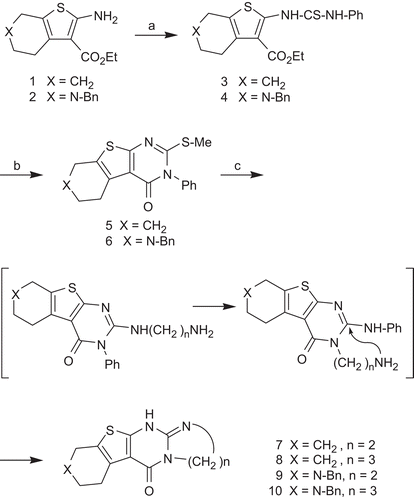
Figure 3. Kinetic model for the interaction of hyperbolic mixed-type inhibitors with Electrophorus electricus acetylcholinesterase. ES, enzyme-substrate complex; ESI, enzyme-substrate-inhibitor complex; EI, enzyme-inhibitor complex.
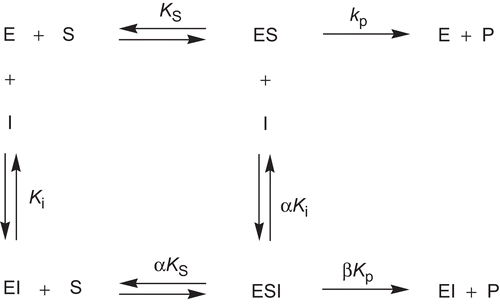
Figure 4. Inhibition of Electrophorus electricus acetylcholinesterase (EeAChE) by compound 10. Plot of the rates versus [I] for the inhibition of EeAChE in 100 mM sodium phosphate, 100 mM NaCl, pH 7.3 with 350 µM 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), 6% acetonitrile and ∼ 0.03 U/mL EeAChE. Rates were obtained by linear regression of the progress curves and are mean values of duplicate experiments. A rate at a substrate concentration of 500 µM in the absence of inhibitor was set 100%. (A) Initial substrate concentration was 250 µM acetylthiocholine (ATCh). The solid line was drawn using the best-fit parameters from a fit according to Equation 1, which gave IC50 = 1.61 ± 0.04 µM and v[I]→∞ = 5.0 ± 0.5%. (B) Initial substrate concentration was 500 µM ATCh. Non-linear regression according to Equation 1 gave IC50 = 1.51 ± 0.07 µM (see ) and v[I]→∞ = 11 ± 1%. (C) Initial substrate concentration was 750 µM ATCh. Non-linear regression according to Equation 1 gave IC50 = 1.39 ± 0.09 µM and v[I]→∞ = 17 ± 2%. (D) Initial substrate concentration was 1000 µM ATCh. Non-linera regression according to Equation 1 gave IC50 = 1.38 ± 0.07 µM and v[I]→∞ = 20 ± 2%.
![Figure 4. Inhibition of Electrophorus electricus acetylcholinesterase (EeAChE) by compound 10. Plot of the rates versus [I] for the inhibition of EeAChE in 100 mM sodium phosphate, 100 mM NaCl, pH 7.3 with 350 µM 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), 6% acetonitrile and ∼ 0.03 U/mL EeAChE. Rates were obtained by linear regression of the progress curves and are mean values of duplicate experiments. A rate at a substrate concentration of 500 µM in the absence of inhibitor was set 100%. (A) Initial substrate concentration was 250 µM acetylthiocholine (ATCh). The solid line was drawn using the best-fit parameters from a fit according to Equation 1, which gave IC50 = 1.61 ± 0.04 µM and v[I]→∞ = 5.0 ± 0.5%. (B) Initial substrate concentration was 500 µM ATCh. Non-linear regression according to Equation 1 gave IC50 = 1.51 ± 0.07 µM (see Table 1) and v[I]→∞ = 11 ± 1%. (C) Initial substrate concentration was 750 µM ATCh. Non-linear regression according to Equation 1 gave IC50 = 1.39 ± 0.09 µM and v[I]→∞ = 17 ± 2%. (D) Initial substrate concentration was 1000 µM ATCh. Non-linera regression according to Equation 1 gave IC50 = 1.38 ± 0.07 µM and v[I]→∞ = 20 ± 2%.](/cms/asset/f7e3d623-891d-4eda-aaee-d8cf80944dce/ienz_a_504674_f0005_b.gif)
Figure 5. Specific velocity plot for the inhibition of Electrophorus electricus acetylcholinesterase (EeAChE) by compound 10. Concentrations of 10 were as follows: open circles, [I] = 1 μM; full circles, [I] = 2 μM; open squares, [I] = 3 μM; full squares, [I] = 4 μM; open triangles, [I] = 5 μM. Data were from duplicate measurements at four different substrate concentrations (250 µM, 500 µM, 750 µM, and 1000 µM). Linear regression according to Equation 2 gave values for vertical intercepts at ([S]/Km)/(1 + [S]/Km) = 0 (a values) and for vertival intercepts at ([S]/Km)/(1 + [S]/Km) = 1 (b values).
![Figure 5. Specific velocity plot for the inhibition of Electrophorus electricus acetylcholinesterase (EeAChE) by compound 10. Concentrations of 10 were as follows: open circles, [I] = 1 μM; full circles, [I] = 2 μM; open squares, [I] = 3 μM; full squares, [I] = 4 μM; open triangles, [I] = 5 μM. Data were from duplicate measurements at four different substrate concentrations (250 µM, 500 µM, 750 µM, and 1000 µM). Linear regression according to Equation 2 gave values for vertical intercepts at ([S]/Km)/(1 + [S]/Km) = 0 (a values) and for vertival intercepts at ([S]/Km)/(1 + [S]/Km) = 1 (b values).](/cms/asset/b61b553d-caba-430a-9df3-413a27041331/ienz_a_504674_f0006_b.gif)
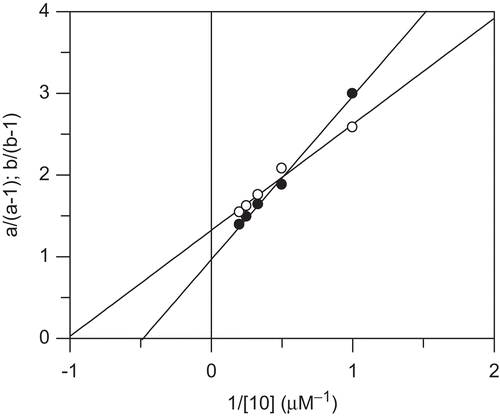
Figure 6. Replots of the specific velocity plot shown in . Values a/(a-1) (full circles) and b(b-1) (open circles) were plotted versus the reciprocal concentrations of inhibitor 10. Linear regression according to Equations 3 and 4 gave values Ki = 2.07 ± 0.19 µM, αKi = 0.98 ± 0.13 µM, β = 0.24.