Figures & data
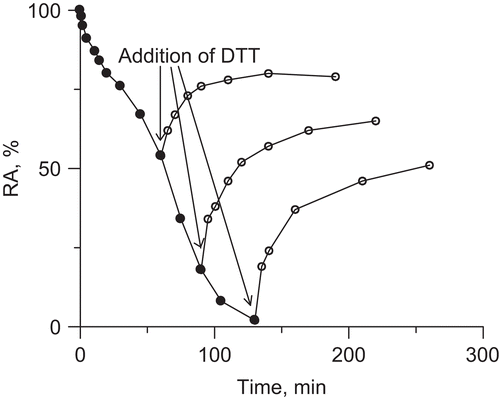
Figure 1. Time courses of urease inactivation (semilogarithmic plots of residual activity (RA) versus incubation time) by: (A) AA in unbuffered system, and (B) acetate buffer. The RA values are reported as percent of the control activity.
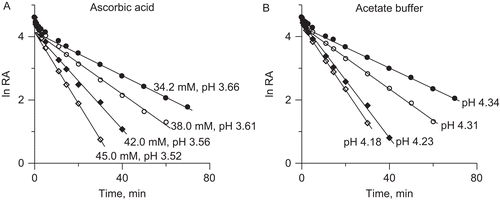
Figure 2. Dose-response curves for 20 min incubation observed in: (A) AA unbuffered solutions, and (B) acetate buffer.
Figure 3. Time courses of urease inactivation (residual activity (RA) versus incubation time) by 10 mM AA and DHA in buffered system (200 mM phosphate buffer pH 7.2) in the absence and presence of 10 μM Fe3+ ions. The RA values are reported as percent of the control activity.
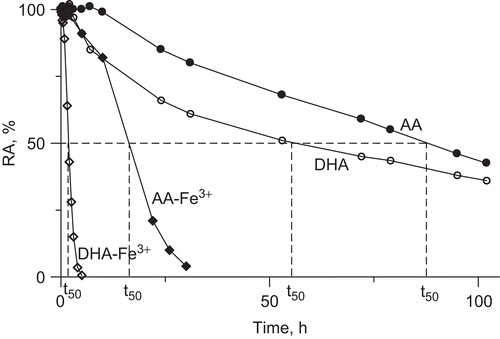
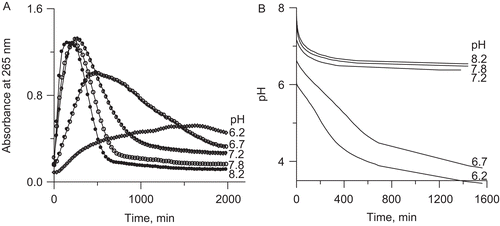
Figure 4. Representative UV-vis spectra of: (A) AA, and (B) DHA during their conversions in 200 mM phosphate buffer pH 7.2, 1 mM EDTA. The insets present kinetic curves of the change in absorbance at 265 nm over time.
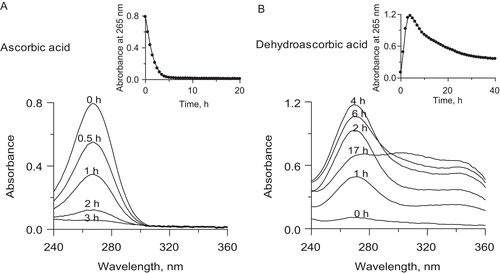
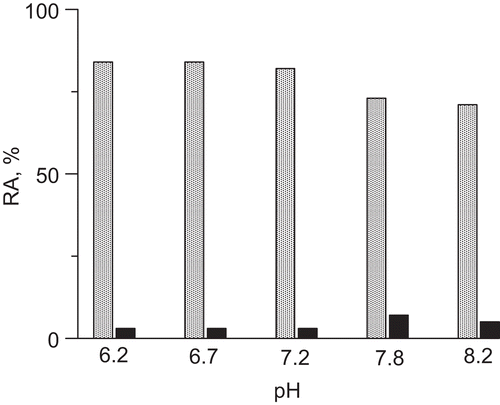
Figure 5. Urease inactivation by 10 mM DHA in the presence of 10 μM Fe3+ in 200 mM phosphate buffer at pHs in the range 6.2–8.2; (A) time courses RA versus time, and (B) semi-logarithmic plots of RA versus time. The RA values are reported as percent of the control activity.
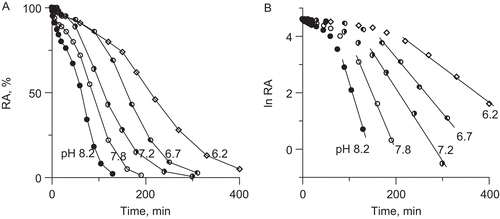
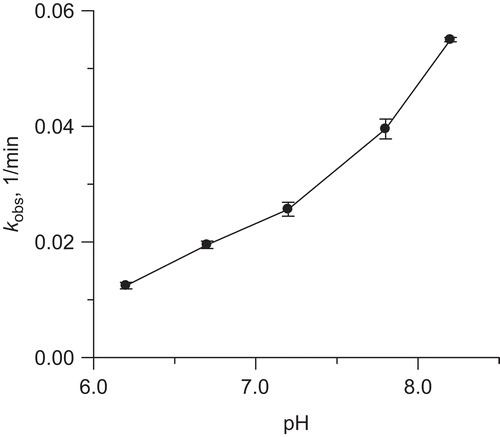
Figure 6. Effect of pH on rate constant of urease inactivation by 10 mM DHA in the presence of 10 μM Fe3+ ions.