Figures & data
Table 1. Analytical and physical data of the complexes.
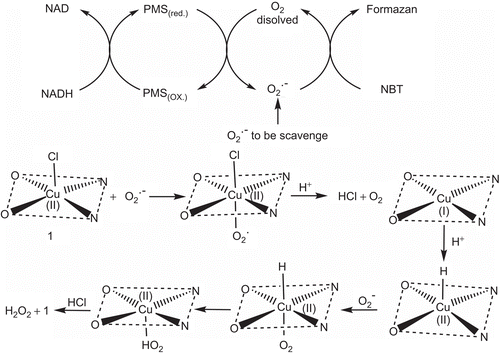
Figure 1. Basic sketch for square pyramidal copper(II) complexes derived from levofloxacin (LFLH); uninegative bidentate OO- donating, and phenanthrolines; neutral bidentate NN- donating ligands.
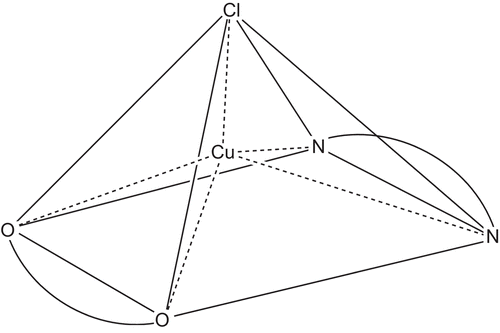
Table 2. Characteristic IR bands (4000–400 cm−1) of LFLH and their complexes.
Figure 2. Fast atom bombardment (FAB)-mass spectrum of complex 1, i.e. [Cu(LFL)(A1)Cl]·5H2O recorded on Jeol SX 102/Da–600 mass spectrophotometer/Data system using Argon/Xenon (6 kV, 10 mA) as the FAB gas at accelerating voltage of 10 kV.
![Figure 2. Fast atom bombardment (FAB)-mass spectrum of complex 1, i.e. [Cu(LFL)(A1)Cl]·5H2O recorded on Jeol SX 102/Da–600 mass spectrophotometer/Data system using Argon/Xenon (6 kV, 10 mA) as the FAB gas at accelerating voltage of 10 kV.](/cms/asset/31150527-3a63-41d8-be35-a00ea2a5911d/ienz_a_506874_f0002_b.gif)
Table 3. Antimicrobial activities of LFLH, phenanthrolines and their complexes in terms of minimum inhibitory concentration (MIC; µM).
Table 4. Binding constant (Kb) and 50% inhibitory concentration (IC50) values of the complexes.
Figure 3. Absorption spectral traces on addition of Herring Sperm DNA to the solution of complex 1 after incubating it for 10 min at room temperature in phosphate buffer at 7.2 pH. (shown by arrow). Inset: plot of [DNA]/(ϵa-ϵf) versus [DNA] for absorption titration of Herring Sperm DNA with complex 1.
![Figure 3. Absorption spectral traces on addition of Herring Sperm DNA to the solution of complex 1 after incubating it for 10 min at room temperature in phosphate buffer at 7.2 pH. (shown by arrow). Inset: plot of [DNA]/(ϵa-ϵf) versus [DNA] for absorption titration of Herring Sperm DNA with complex 1.](/cms/asset/130eb04a-a0a0-4b88-b3ec-de3fc604be6a/ienz_a_506874_f0003_b.gif)
Figure 4. Effect of increasing amount of ethidium bromide (EtBr), levofloxacin (LFLH) and complexes on the relative viscosity of Herring Sperm DNA at 27 ± 0.1°C.
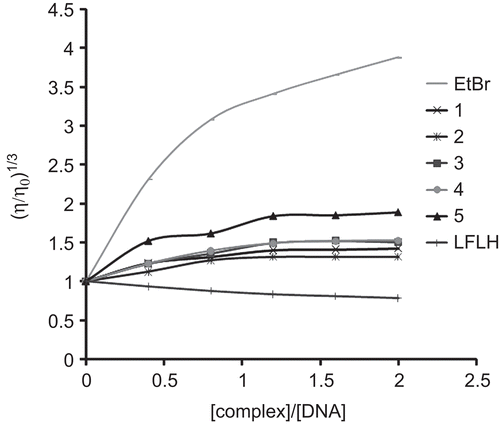
Table 5. Percentage of all the three forms of plasmid separated on agarose gel, when subjection to different synthesized complexes at 200 µM concentration.
Figure 5. Gel electrophoresis diagram showing the cleavage of SC pUC19 DNA(300 µg/mL) with as series of copper(II) complex (200 µM) in a final volume of 15 µL, incubated at 37°C, using 1% agarose gel, at 50 mV for 1.5 h. Lane 1, DNA control; Lane 2, DNA + CuCl2·2H2O; Lane 3, DNA + LFLH; Lane 4, DNA + [Cu(LFL)(A1)Cl].5H2O; Lane 5, DNA + [Cu(LFL)(A2)Cl].5H2O; Lane 6, DNA + [Cu(LFL)(A3)Cl].5H2O; Lane 7, DNA + [Cu(LFL)(A4)Cl].5H2O; Lane 8, DNA + [Cu(LFL)(A5)Cl].5H2O.
![Figure 5. Gel electrophoresis diagram showing the cleavage of SC pUC19 DNA(300 µg/mL) with as series of copper(II) complex (200 µM) in a final volume of 15 µL, incubated at 37°C, using 1% agarose gel, at 50 mV for 1.5 h. Lane 1, DNA control; Lane 2, DNA + CuCl2·2H2O; Lane 3, DNA + LFLH; Lane 4, DNA + [Cu(LFL)(A1)Cl].5H2O; Lane 5, DNA + [Cu(LFL)(A2)Cl].5H2O; Lane 6, DNA + [Cu(LFL)(A3)Cl].5H2O; Lane 7, DNA + [Cu(LFL)(A4)Cl].5H2O; Lane 8, DNA + [Cu(LFL)(A5)Cl].5H2O.](/cms/asset/522c5795-888e-4405-b7c8-bcf4d3e80508/ienz_a_506874_f0005_b.gif)