Figures & data
Table 1. Km and Vmax values for five expressed human UGTs*
Figure 2. The enzyme kinetics of expressed human UGT1A1, 2B7, 1A4, and 1A6. UGT1A1-mediated β-estradiol-3-glucuronidation (A), UGT2B7-mediated diclofenac-glucuronidation (B), UGT1A4-mediated midazolam-N-glucuronidation (C), and UGT1A6-mediated 1-naphthol-glucuronidation (D) were fitted with Michaelis–Menten model and Eadie–Hofstee plots, respectively.
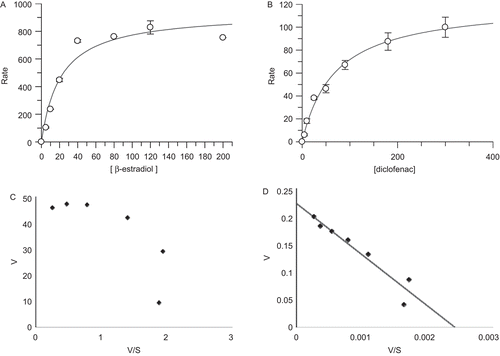
Table 2. Determination of the IC50 values*.
Figure 3. The inhibition plots of expressed human UGT1A4-mediated midazolam-N-glucuronidation by α-naphthoflavone (A), and paclitaxel (B); UGT1A6-mediated 1-naphthol-glucuronidation by midazolam (C); UGT1A4-mediated midazolam-N-glucuronidation by cyclosporine A (D); UGT1A1-mediated β-estradiol-3-glucuronidation by ketoconazole (E). Their inhibition IC50 values were calculated to be 23 ± 6, 4.9 ± 0.3, 23 ± 1, 23 ± 7, and 4.1 ± 0.8 µM, respectively.
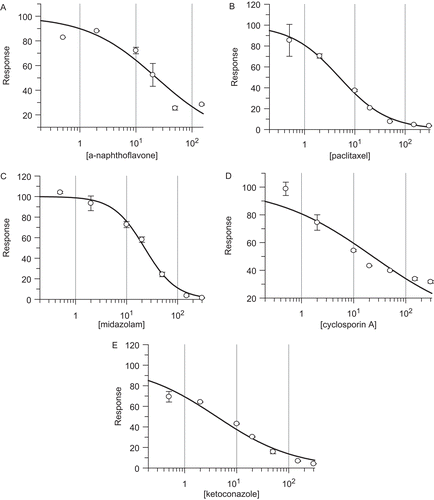
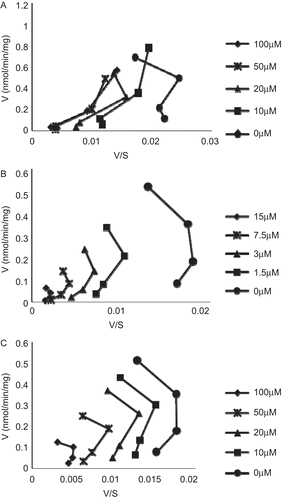
Figure 4. Eadie–Hofstee plots for inhibitions kinetics of expressed human UGT1A1-mediated β-estradiol-3-glucuronidation by paclitaxel (A), ketoconazole (B), and midazolam (C). The concentrations of inhibitors were as shown in the figures, and the substrate concentrations were set at 5, 10, 20, and 40 µM for β-estradiol.