Figures & data
Table 1. Analytical and physical data for complexes.
Table 2. Main IR spectral bands for complexes.
Figure 1. TGA curve of {weight (%) vs temperature (°C)} (A) [Co(C11H17N3S)Cl2] and (B) [Cu (C11H17N3S)Cl2].
![Figure 1. TGA curve of {weight (%) vs temperature (°C)} (A) [Co(C11H17N3S)Cl2] and (B) [Cu (C11H17N3S)Cl2].](/cms/asset/97720107-1d9a-44dc-bcfc-b8130c24a073/ienz_a_518966_f0001_b.gif)
Table 3. Fragmented molecular ions vs m/z values of following metal complexes.
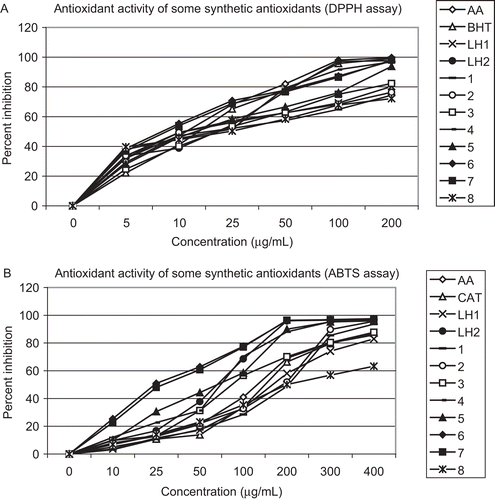
Table 4. Antioxidant activity of metal complexes and ligands (DPPH assay).
Table 5. Antioxidant activity of metal complexes and ligands (ABTS assay).
Table 6. IC50 values of test compounds (µg/mL).
![Figure 2. Structural formula for the complexes (A) [M(LH)Cl2] and (B) [M(LH)2Cl2].](/cms/asset/bb4af39f-e118-40d7-9f8b-3fbdd47c239d/ienz_a_518966_f0002_b.gif)