Figures & data
Table 1. Analytical data for compounds 1–16.
Scheme 1. Reagent and conditions: (a) EtOH/warm, room temperature, 2–10 days and (b) MeOH-CHCl3, H2NNHCSNHPh/H+, reflux, 90–100°C 4 h.
Table 2. Correlations in the H,H-COSY and NOESY spectra of compound 9.
Table 3. Correlations in the HSQC spectrum of compound 9.
Figure 1. Correlations between the protons that are in “W” arrangements, from the H,H-COSY spectrum of compound 9.
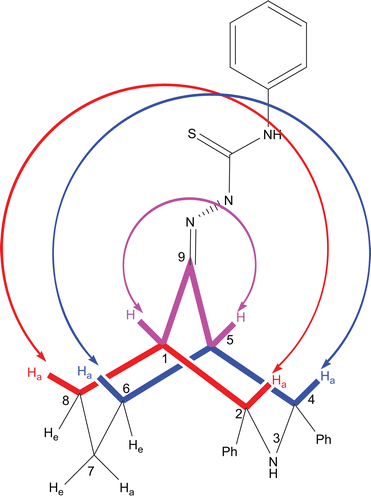
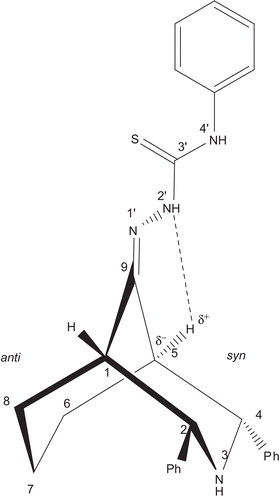
Figure 3. Twin-chair conformation with equatorial orientation of the phenyl groups at C-2 and C-4; supported by the NOE observed in the NOESY spectrum of compound 9. For clarity, the NOE between the ortho and ring protons is shown by red colour lines.
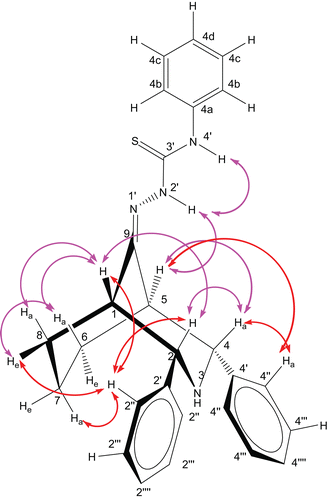
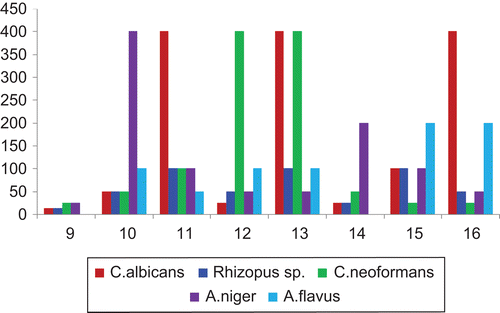
Table 4. Antibacterial activity of compounds 9–16 against selected bacterial strains (MIC in μg/mL).
Table 5. Antifungal activity of compounds 9–16 against selected fungal strains (MIC in μg/mL).
![Figure 4. Comparison of potency of compounds 9–16 with ciprofloxacin (as standard) against bacterial strains from serial dilution method. Scheme 1: Schematic diagram showing the synthesis of 2,4-diaryl-3-azabicyclo[3.3.1]nonan-9-one 4′-phenylthiosemicarbazones.](/cms/asset/4599d44a-f215-47e7-8db6-4af13cac0b1c/ienz_a_525508_f0005_b.gif)