Figures & data
Figure 1. Kinetics of di-eosin oxidized glutathione reduction into fluorescent E-GSH catalyzed by protein disulphide isomerase in the presence of various concentrations (0–10−4 M) of melatonin, auxin, serotonin or metoprolol.
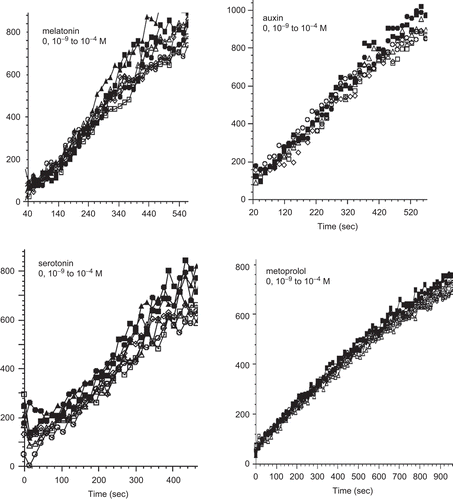
Figure 2. Kinetics of di-eosin oxidized glutathione reduction into fluorescent E-GSH catalyzed by protein disulphide isomerase in the presence of various concentrations (0–10−4 M) of bisphenol A. BPA, bisphenol A; RFU, relative fluorescence unit.
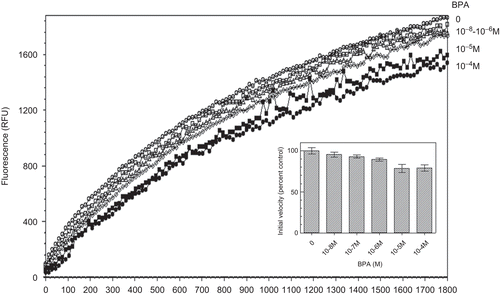
Figure 3. Effect of increasing concentrations of bacitracin (BAC) on protein disulphide isomerase (PDI) reductase activity. Left panel: kinetics of di-eosin oxidized glutathione reduction into fluorescent E-GSH catalyzed by PDI in the presence of various concentrations (0–10−4 M) of BAC. Right panel: initial velocity of PDI reducing activity as a function of BAC concentration relative to the velocity in the absence of bacitracin taken as control (n = 3). RFU, relative fluorescence unit.
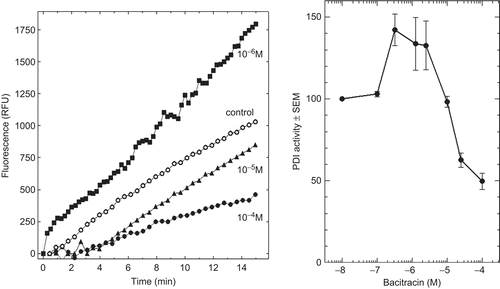
Figure 4. Kinetics of di-eosin oxidized glutathione reduction into fluorescent E-GSH catalyzed by protein disulphide isomerase in the presence of various concentrations (0, 10−4, 10−3 M) of fluoxetine (FLX). The figure also shows the effect of 10% ethanol alone as also present with 10−3 M FLX.
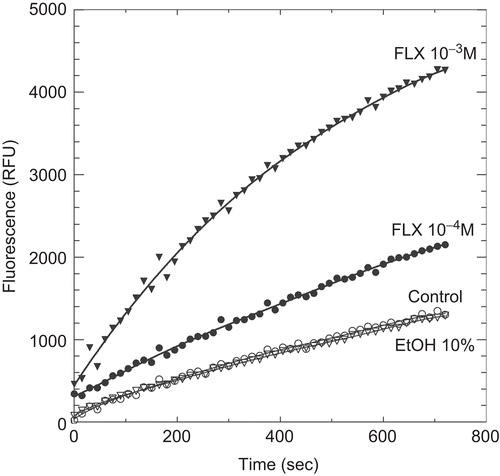
Figure 5. Kinetics of di-eosin oxidized glutathione reduction into fluorescent E-GSH catalyzed by protein disulphide isomerase in the presence or absence of 0.5 M ammonium sulphate. RFU, relative fluorescence unit.
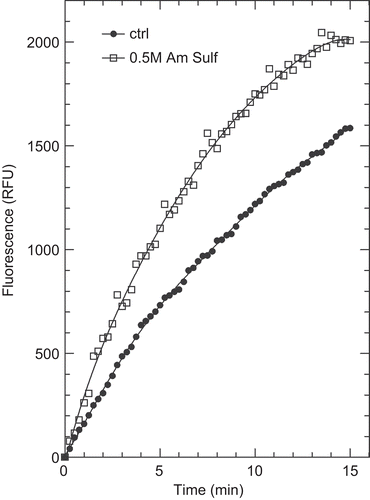
Figure 6. Inhibition of protein disulphide isomerase (PDI) reductase activity by diethylstilbestrol (DES) in the presence of a potentiating concentration of 19-nortestosterone (19-NT). Left panel: kinetics of di-eosin oxidized glutathione reduction into fluorescent E-GSH catalyzed by PDI in the presence of 10−5 M of either DES or 19-NT or in the presence of 10−5 M 19-NT together with various concentrations (10−9, 10−7, 10−5 M) of DES. Right panel: initial velocity of PDI reducing activity in the presence of either DES or 19-NT alone or in combination (data from left panel). RFU, relative fluorescence unit.
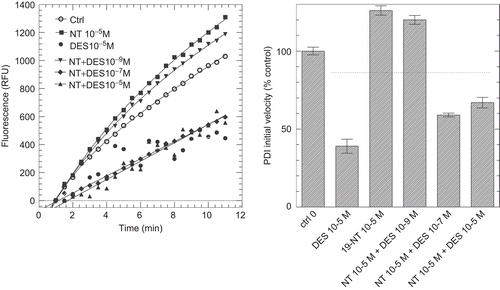
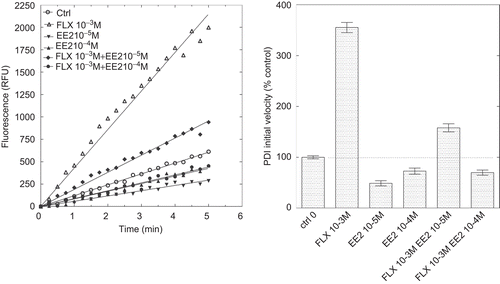
Figure 7. Inhibition of protein disulphide isomerase (PDI) reductase activity by ethynylestradiol (EE2) in the presence of a potentiating concentration of fluoxetine (FLX). Left panel: kinetics of di-eosin oxidized glutathione reduction into fluorescent E-GSH catalyzed by PDI in the presence of 10−3 M FLX or 10−5 or 10−4 M EE2 alone or in the presence of 10−3 M FLX. Right panel: initial velocity of PDI reducing activity in the presence of either FLX or EE2 alone or in combination. RFU, relative fluorescence unit.