Figures & data
Figure 1. Time course and protein concentration curves for p-Nph-5′-TMP hydrolysis. Time course in the soluble fraction was performed with 90 µg of protein; 30 µg of protein was used in microsomal fraction. In relation to protein concentration curves were used 40 and 6 min for p-Nph-5′-TMP hydrolysis in soluble and microsomal fractions, respectively. The plots are representative of three independent experiments for each fraction.
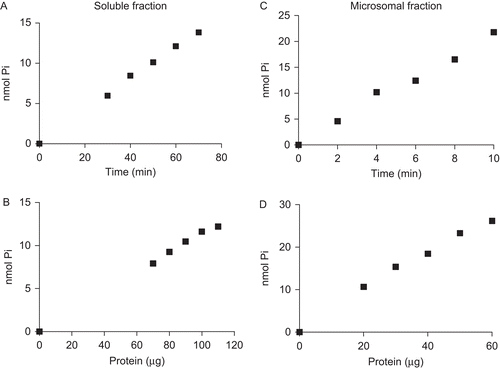
Figure 2. Divalent cations dependence on p-Nph-5′-TMP hydrolysis. Hydrolysis of p-Nph-5′-TMP by rat cardiac soluble (A) and microsomal (B) fractions were analysed in the absence of cations (Control), in the presence of 0.025, 0.05 and 0.1 mM EDTA and in the presence of 2.0–8.0 mM Ca2+ or Mg2+. Bars represent means ± SD of three independent experiments. Results are expressed as nmol p-nitrophenol/min/mg of protein. *Significantly different from respective control group (P < 0.05).
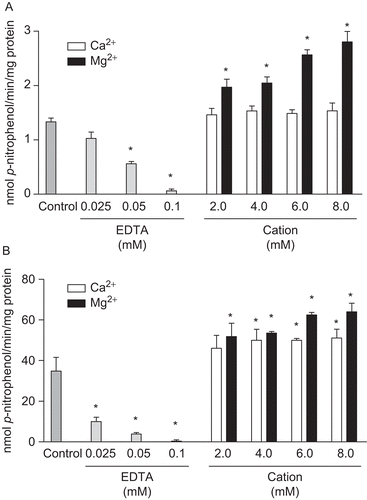
Figure 3. Effect of pH on p-Nph-5′-TMP hydrolysis in rat cardiac soluble (A) and microsomal (B) fractions. Enzyme activity was determined as described in “Materials and methods” using a mixture of the following buffers: Tris and glycine (pH range 8.0 to 10.0). Data represent means ± SD of three different experiments. Results are expressed as nmol p-nitrophenol/min/mg of protein.
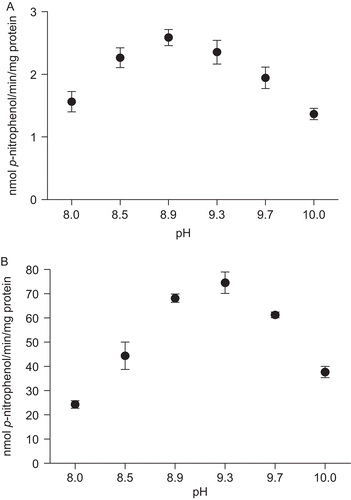
Figure 4. Eadie–Hofstee plot for p-Nph-5′-TMP hydrolysis. The nucleotide hydrolysis as function of substrate concentration from rat cardiac soluble (A) and microsomal (B) fractions is shown in the insets. The mean KM values calculated for p-Nph-5′-TMP hydrolysis were found to be 118.5 ± 27.2 µM and 91.9 ± 12.4 µM, respectively, and Vmax value calculated in soluble and microsomal fractions were 2.52 ± 0.1 and 113.87 ± 21.1 nmol p-nitrophenol/min/mg, respectively. Data are expressed as mean ± SD, n = 4.
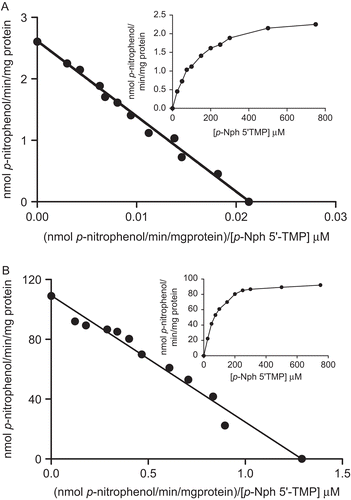
Table 1. Effect of distinct compounds on p-nitrophenyl-5′-TMP hydrolysis from rat cardiac soluble and microsomal fractions.