Figures & data
Table 1. Physical measurements and analytical data of metal(II) complexes, (1)–(8).
Table 2. Conductivity, magnetic and spectral data of metal(II) complexes, (1)–(8).
Table 3. Antibacterial study (concentration used 1 mg/mL of DMSO) of ligands (L1) and (L2) and metal(II) complexes (1)–(8).
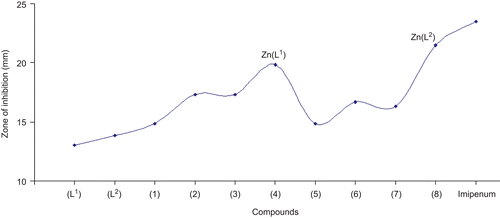
Figure 1. Comparison of antibacterial activity of ligands (L1) and (L2) and metal(II) complexes (1)–(8).
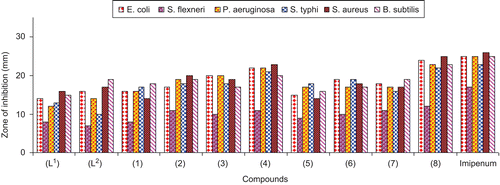
Table 4. Antifungal study (concentration used 200 µg/mL) of ligands (L1) and (L2) and metal(II) complexes (1)–(8).
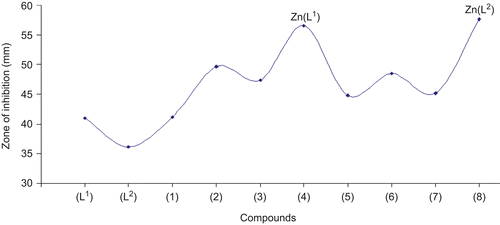
Figure 3. Comparison of antifungal activity of ligands (L1) and (L2) and metal(II) complexes (1)–(8).
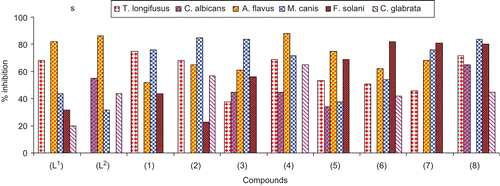
Table 5. Minimum inhibitory concentration (M/mL) of the selected compounds (2), (4) and (8) against selected bacterial strains.
Table 6. Brine shrimp study of the ligands (L1)–(L2) and their metal(II) complexes (1)–(8).
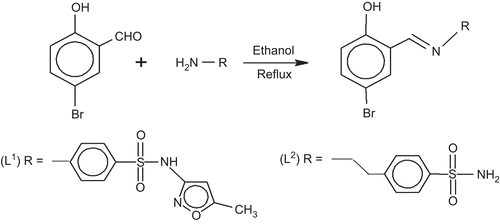
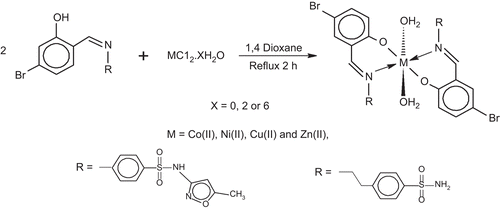
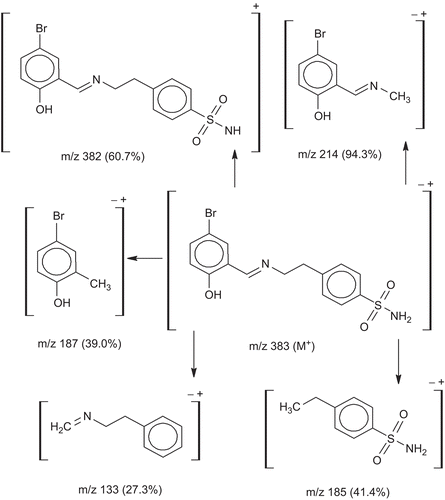
![Figure 6. The proposed fragmentation pattern of complex [Cu(L2-H)2(H2O)2].](/cms/asset/9d1183ff-4453-4e62-8518-2abec7f97031/ienz_a_574623_f0008_b.gif)