Figures & data
Figure 1. The chromatograms of the reaction mixtures of the 3-(3,4-dihydroxyphenyl)-l-alanine (DOPA) oxidation catalyzed by phenoloxidase (PO) in the (A) absence and (B) presence of phenylthiourea (PTU). Incubated mixtures contained 1 mM DOPA, 20 U/mL PO and 1 mM PTU (in the mixture B) in 50 mM phosphate buffered saline (PBS) pH 7.4. Incubation time was 50 min.
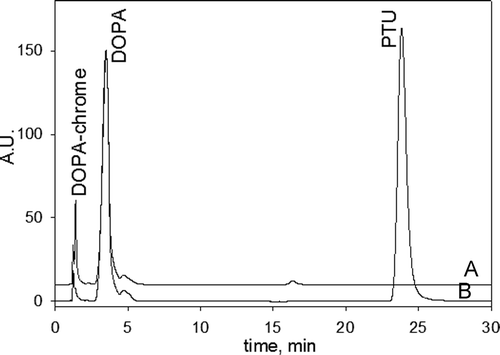
Figure 2. (A) The kinetic curves of the oxidation of 3-(3,4-dihydroxyphenyl)-l-alanine (DOPA) catalyzed by phenoloxidase (PO) in the presence of the different concentrations of phenylthiourea (PTU) and (B) the dependence of the reaction rate from the initial linear part of kinetics on PTU concentration. The conditions were as follows: 1 mM DOPA, 20 U/mL PO and various concentration of PTU (0–20 µM) in 50 mM phosphate buffered saline (PBS) pH 7.4.
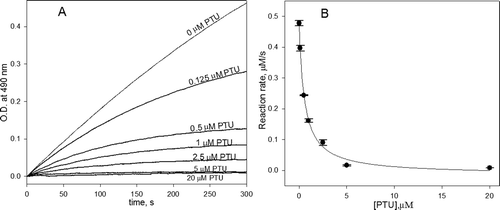
Figure 3. The kinetic curves for checking the reversibility of the inhibition of phenoloxidase (PO) by phenylthiourea (PTU). PO (200 U/mL) was incubated with PTU (200 µM) for 5 min at 25°C. After that, 3-(3,4-dihydroxyphenyl)-l-alanine (DOPA) was added to a final concentration of either (A) 1 mM or (B) 9 mM. The final concentrations of PO and PTU were 20 U/mL and 20 µM, respectively. The long dash line is a linear regression fit of reaction rate data.
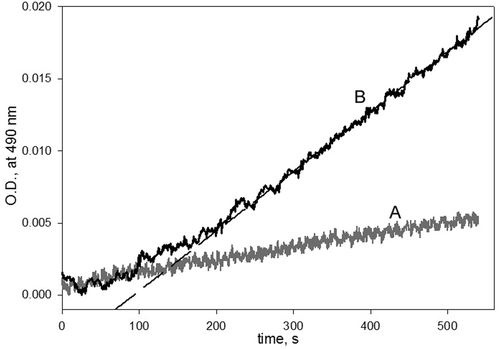
Figure 4. (A) The dependences of the reaction rates of 3-(3,4-dihydroxyphenyl)-l-alanine (DOPA) oxidation catalyzed by phenoloxidase (PO; 20 U/mL) on substrate concentration in the absence (•) and in the presence of phenylthiourea (PTU): 0.125 µM (▪), 0.5 µM (▾), 2.5 µM (♦). —, nonlinear regression fit of data to Michaelis–Menten equation for competitive inhibition. The linearizations of these dose-dependent curves: (B) the Lineweaver-Burk plot, (C) the Hanes-Woolf plot and (D) the Eadie-Hofstee plot. The symbol meanings are the same that in (A).
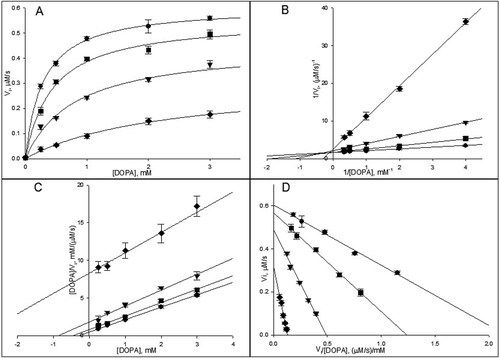
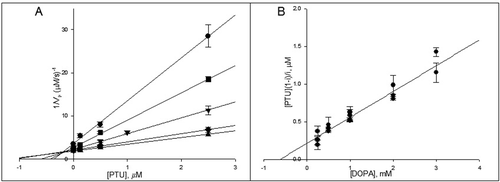
Figure 5. The Dicson (A) and the one-curve method (B) linearizations of the data presented in A. The symbols meanings are (•) 0.25 mM, (▪) 0.5 mM, (▾) 1 mM, (♦) 2 mM and (▴) 3 mM of 3-(3,4-dihydroxyphenyl)-l-alanine (DOPA).
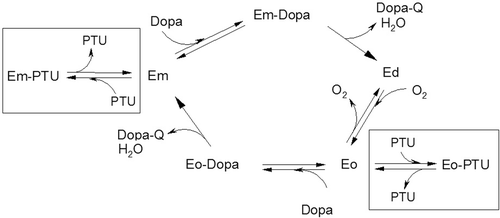
Scheme 1. Simplified kinetic scheme which describes the effect of phenylthiourea (PTU) on the 3-(3,4-dihydroxyphenyl)-l-alanine (DOPA) oxidation catalyzed by phenoloxidase (PO). The processes in the presence of PTU are marked by frames. The enzymatic forms of PO are as follows: Em− met-phenoloxydase (with Cu(II)-Cu(II) in the active site), Ed− deoxy-phenoloxydase (with Cu(I)-Cu(I) in the active site), Eo− oxy-phenoloxydase (with Cu(II)-O2-Cu(II) in the active site).