Figures & data
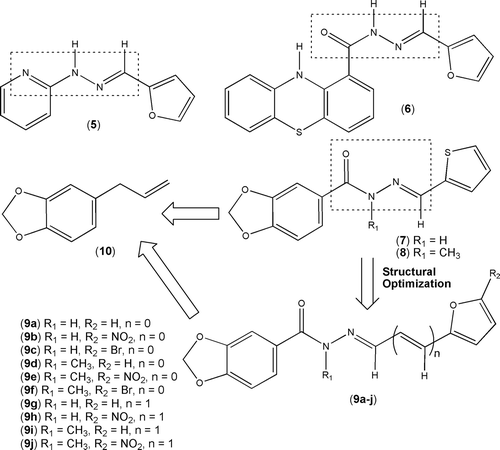
Figure 2. Design concept of new functionalized furylydene 1,3-benzodioxolyl-N-acylhydrazine derivatives 9a–f and the vinylogous analogues 9g–j.
Scheme 1. Reaction conditions: a) I2, KOH, MeOH, rt, 6 h, 90%; b) NH2NH2.H2O 80%, EtOH, reflux, 3.5 h, 84%; c) Functionalized 2-furyl-(CH=CH)n-CHO (14a–e), EtOH, rt, 30 min, see ; d) K2CO3, acetone, MeI, 40°C, 24 h, see supplementary Table S1.
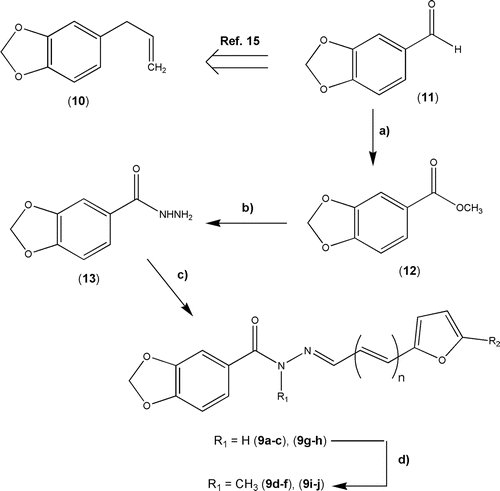
Table 1. Effect of functionalized 2-furyl N-acylhydrazone derivatives 9a–j on platelet aggregation induced by arachidonic acid (AA), collagen, and ADP in rabbit platelet-rich plasma.
Figure 3. McLafferty rearrangement peak in the mass spectra of (E)-(2-furfurylidene) 3,4-methylenedioxybenzoylhydrazine derivative 9a.
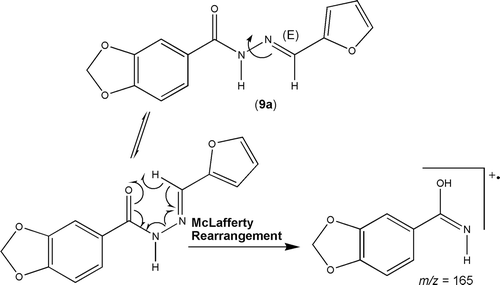
Table 2. Effect of ASA and functionalized 2-furyl N-acylhydrazone derivatives 9a, 9c–e, and 9i–j on platelet aggregation induced by ADP (3 µM) in human platelet-rich plasma.
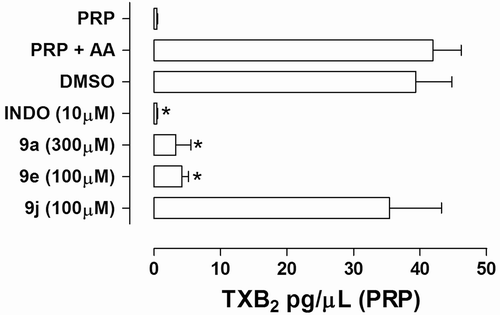
Figure 4. Effect of NAH derivatives 9a, 9e, and 9j on the TXB2 production in arachidonic acid (500 µM)-induced platelet aggregation in human PRP. Results are expressed in terms of mean ± SEM (n = 3–5 independent experiments). *P < 0.05 (ANOVA one-way; Dunnet posttest); N-acylhydrazone derivatives 9 were incubated with plasma-rich platelet 5 min before the addition of AA; The vehicle concentration in the samples does not exceed 0.3%; INDO = indomethacin.