Figures & data
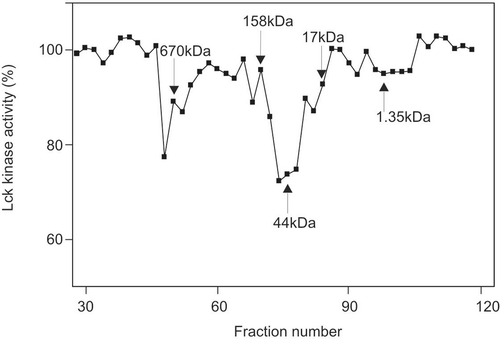
Figure 1. GST-Lck inactivation profile of DEAE Sepharose Fast Flow column eluted proteins. Two microliters of fraction sample was incubated with GST-Lck for 20 min followed by 20 min of kinase assay against cdc2(6-20) peptide substrate. This is a representative profile of three reproducible experiments.
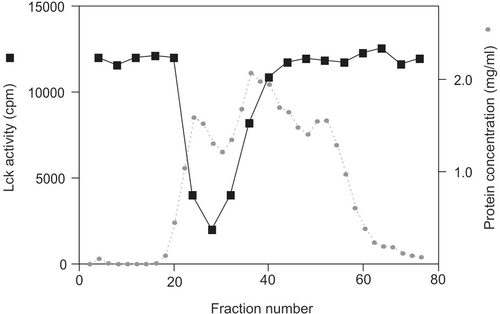
Figure 2. Specificity of inhibitory factor. Buffer or inhibitor sample was incubated with each indicated kinase for 20 min followed by assay of kinase activity against corresponding substrates. This result is representative of three reproducible experiments.
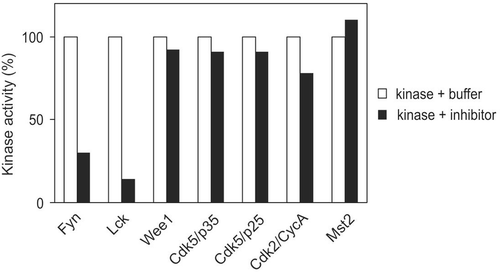
Figure 3. Possibility of phosphatase. Buffer or inhibitor sample was assayed for their ability to dephosphorylate phosphor-cdc2(6-20) or inhibit kinase activity of GST-Fyn and GST-Lck. The amount of kinase and inhibitor were identical in phosphatase test and kinase inhibition test. Both the time for substrate dephosphorylation (phosphatase test) and preincubation (kinase inhibition test) were 20 min. This result is representative of three reproducible experiments.
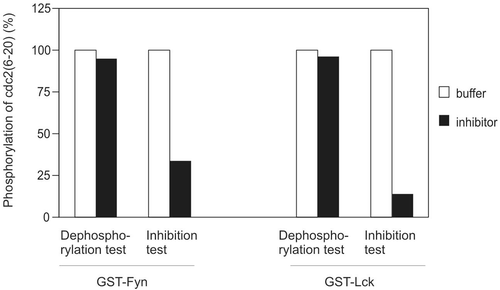
Figure 4. Possibility of ATPase. Buffer, inhibitor, or positive ATPase sample was assayed for their ability to inhibit substrate phosphorylation by GST-Lck or to hydrolyse ATP. The amount of kinase, inhibitor, and positive ATPase were identical in the kinase inhibition test and the ATPase test. The time of preincubation for both the kinase inhibition test and ATPase test were 20 min. This result is representative of three reproducible experiments.
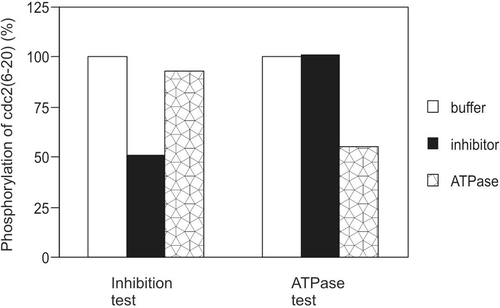
Figure 5. Western blot of protein kinases treated with the inhibitory sample. Protein kinases were incubated with lysis buffer or inhibitory sample for 20 min and then incubated for 20 min in the presence of ATP and cdc2(6-20) peptide substrate. Reaction was terminated by heat denaturation. (A) Purified bovine Fyn with buffer A (lanes 1 and 3) and inhibitor sample (lanes 2 and 4). The amount of Fyn protein sample loaded was 500 ng (lanes 1 and 2) and 50 ng (lanes 3 and 4). The Fyn kinase activity after incubation with inhibitor sample was 30% of that treated with buffer A. (B) GST-Lck with buffer A (lanes 1 and 2) and inhibitor sample (lanes 3 and 4). The amount of GST-Lck protein loaded on each track was 40 ng (lanes 1 and 3) and 13 ng (lanes 2 and 4). The GST-Lck kinase activity treated with inhibitor sample was 8% of that treated with buffer A. This result is representative of three reproducible experiments.
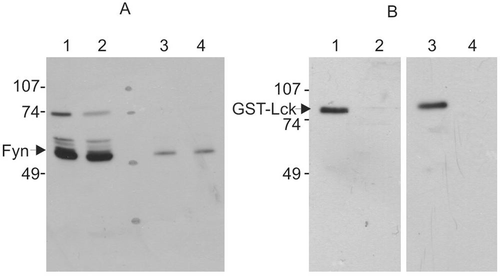
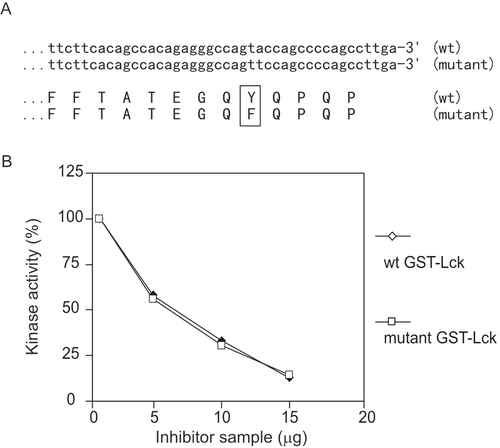
Figure 6. CSK test. (A) Alignment of nucleotide sequences at 3′ of wild-type and mutant Lck and corresponding amino acid sequences at C-terminus. The full-length sequences were obtained and identical except at the 3′ (C-terminus), which is shown. (B) Dose-dependence comparison of inhibition on wild-type and mutant GST-Lck. This result is representative of three reproducible experiments.