Figures & data
Table 1. Tpl2 kinase inhibition data of quinoline-3-carbonitrile and their analogues.
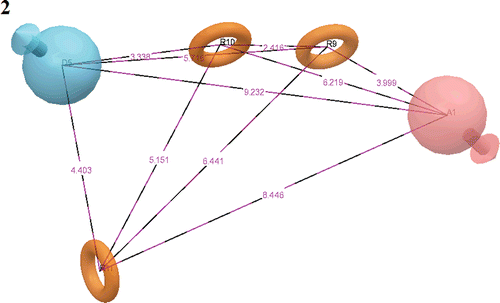
Figure 1. Common pharmacophore for active ligands (three aromatic rings (grey circle)), one H-donor (blue sphere with single arrow) and one acceptor (pink sphere with one arrow).
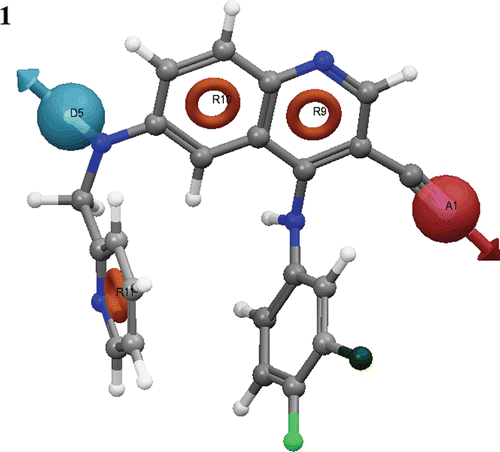
Table 2. The scores of different parameters of the ADRRR hypothesis.
Table 3. PLS statistical parameters of the selected 3D-QSAR model.
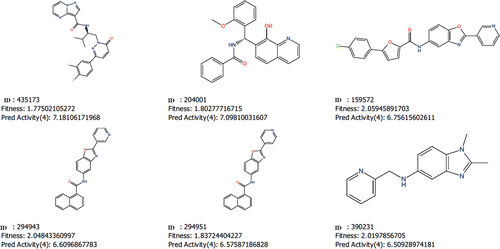
Table 4. Fitness and predicted activity data for the test and training set of compounds.
Figure 4. Fitness graph between observed activity vs. Phase predicted activity for training and test set compounds.
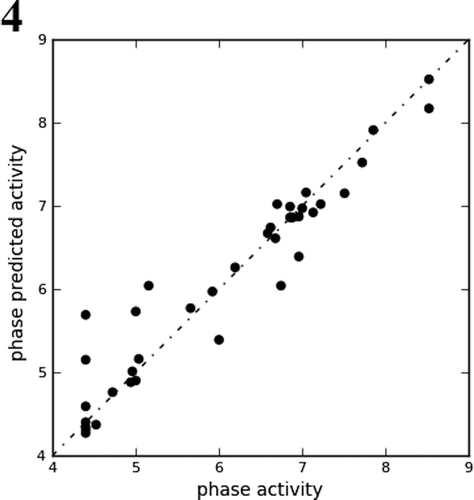
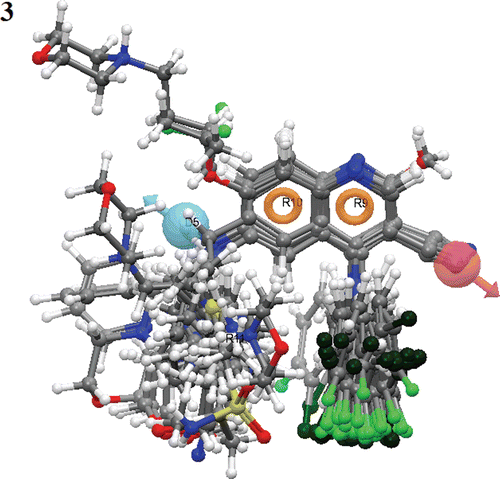
Figure 5. QSAR visualization of various substituents effect: (A) Electron withdrawing feature, (B) Hydrogen-bond donor, (C) Hydrophobic features, (D) Positive ionic and (E) Negative ionic.
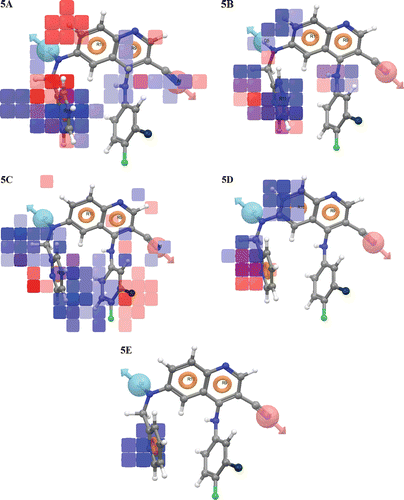
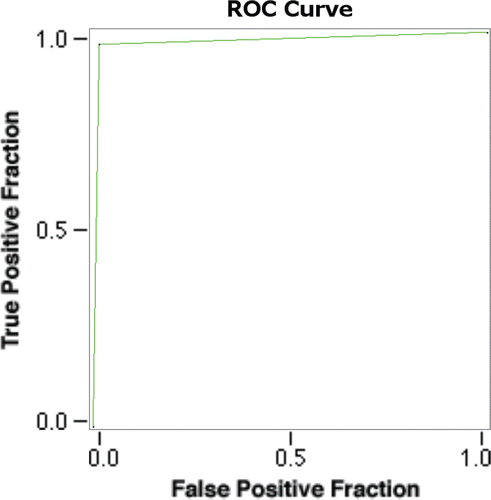
Table 5. The ROC and a few performance measures derived from the number of true positives, true negatives, false positives and false negatives by confusion matrix.