Figures & data
Figure 1. Chemical structures of standard antioxidants (BHA, BHT, α-tocopherol and trolox), majdine and isomajdine from Vinca herbacea Waldst. and Kit.
Figure 2. (A) Fe3+-Fe2+ reductive potential of different concentrations (10–30 g/mL) of majdine (r2:0.933), isomajdine (r2:0.933) and reference antioxidants BHA, BHT, α-tocopherol and trolox using spectrophotometric detection of the Fe+3-Fe+2 transformation. (B) Cupric ions (Cu2+) reducing ability (Cuprac method) of different concentrations (10–30 g/mL) of majdine (r2: 0.9330), isomajdine (r2: 0.9330), BHA, BHT, α-tocopherol and trolox using spectrophotometric detection of the Cu+2-Cu+ transformation. (BHA: Butylated hydroxyanisole, BHT: Butylated hydroxytoluene).
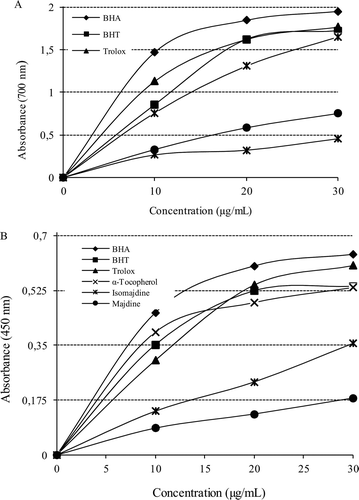
Table 1. Comparison of IC50 values of ABTS•+ scavenging, DMPD•+ scavenging and ferrous ion (Fe2+) chelating activity of majdine, isomajdine and standard antioxidant compounds such as BHA, BHT, α-tocopherol and trolox (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene, ABTS•+:2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) radicals, DMPD•+: N,N-dimethyl-p-phenylenediamine radicals).
![Figure 3. WST-1 assay of the groups. [H2O2: 1 mM of hydrogen peroxide. Iso: isomajdine and majd: majdine in µM concentrations. *p < 0.05, **p < 0.01 with respect to control group (Con), WST-1: water-soluble tetrazolium salt].](/cms/asset/1b3885b7-9b55-453b-b471-caab639c2fa8/ienz_a_604318_f0003_b.gif)