Figures & data
Table 1. Quantitative data of LDH and esterase activities within non-denaturing IEF, non-denaturing stacking gel containing inhibitors (oxamate for LDH activity and acrinol for esterase activity) and non-denaturing separation gel after the separation of the cytosolic proteins in the mouse liver by non-denaturing IEF.
Figure 1. LDH activities in the non-denaturing stacking gel containing 0.2% oxamate (a) and in the non-denaturing separation gel (b) after the separation of the cytosolic proteins in the mouse liver by non-denaturing IEF. LDH activity spots are indicated by arrows.
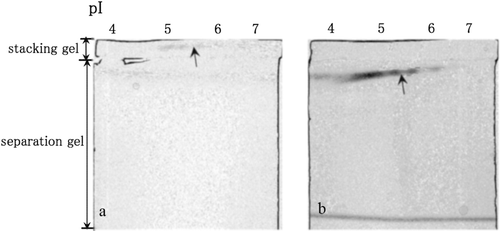
Figure 2. Esterase activities in the non-denaturing stacking gel containing 0.1 M Tris–HCl (pH 6.8) (a), in the non-denaturing stacking gel containing 0.1 mM acrinol and 0.1 M acetate buffer (pH 4.8) (b) and in the non-denaturing separation gel (c) after the separation of cytosolic proteins in the mouse liver by non-denaturing IEF. Esterase activity spots are indicated by arrowheads.
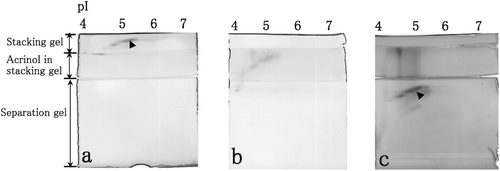
Figure 3. After separation by non-denaturing 2-DE and electroblotting onto membrane, LDH activities staining when the membrane was treated by 0.2% oxamate (a), and washed by Tris–HCl solution (b). After separation by non-denaturing 2-DE and electroblotting onto membrane, esterase activity staining when the membrane was treated by 0.1 mM acrinol (c), and washed by aspartic acid solution (d).
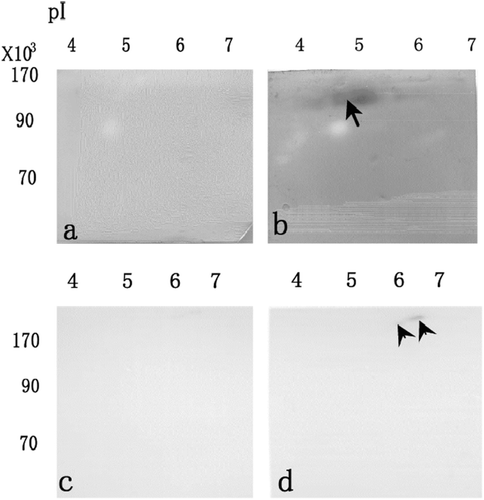
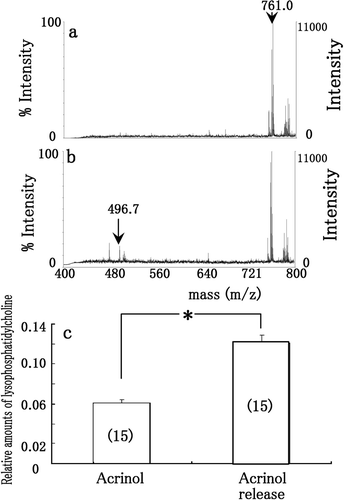
Figure 4. MALDI-TOF MS spectra of phosphatidylcholine and lysophosphatidylcholine when phosphatidylcholine was incubated with the esterase on membrane (arrow in 3d) treated with 0.1 mM acrinol (a), and washed by aspartic acid solution (b) after separation by non-denaturing 2-DE and electroblotting onto membrane. The relative amounts of lysophosphatidylcholine (m/z = 496.7) (c) when pure phosphatidylcholine was applied to esterase on membrane treated with 0.1 mM acrinol (acrinol in c), and washed by aspartic acid solution (acrinol release in c) after separation by non-denaturing 2-DE and electroblotting onto membrane. Each datum indicates mean standard error obtained from 15 individual measurements (number of measurements in c).