Figures & data
Scheme 1. Reagents and reaction conditions: (i) ethanol, NH2NH2·H2O, ref lux, 1 h; (ii) arylsulfonyl chloride, pyridine, ref lux, 4 h; (iii) anhyd. K2CO3, dry acetone, RNCO, ref lux, 18 h; (iv) anhyd. K2CO3, dry acetone, RNCS, ref lux, 10 h; (v) ethyl bromoacetate, Na acetate, ethanol, ref lux 2 h; (vi) ethanol, phenacyl bromide, Na acetate, ref lux 2 h; (vii) approp. aldehyde, ref lux, 3 h; (viii) thioacetic acid, dry dioxan, ref lux 12 h.
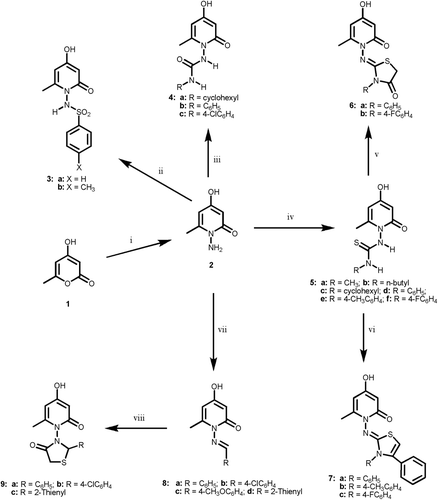
Scheme 2. Reagents and reaction conditions: (i) ethanol, sulfanilamide, ref lux, 4 h; (ii) anhyd. K2CO3, dry acetone, approp. RNCO or RNCS, ref lux, 10–18 h; (iii) NaNO2/CH3COOH, stirring, 2 + 2 h; (iv) arylsulfonyl chloride, pyridine, ref lux, 4 h; (v) anhyd. K2CO3, dry acetone, RNCO, ref lux, 18 h; (vi) anhyd. K2CO3, dry acetone, RNCS, ref lux, 10 h.
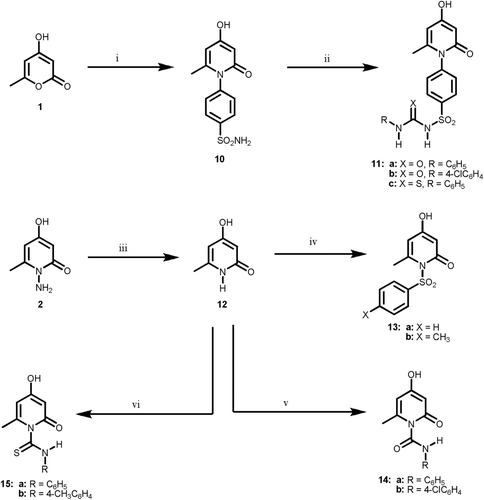
Scheme 3. Reagents and reaction conditions: (i) sulfanilamide, ethanol, ref lux, 4 h; (ii) RCHO, ethanol, ref lux, 3 h; (iii) HSCH2COOH, dry dioxan, ref lux, 12 h; (iv) RNCO, anhyd. K2CO3, dry acetone, ref lux 18 h; (v) RNCS, anhyd. K2CO3, dry acetone, ref lux 10 h; (vi) ethyl bromoacetate, anhyd. NaOAc, abs. ethanol, ref lux, 2 h; (vii) phenacyl bromide, anhyd. NaOAc, abs. ethanol, ref lux, 2 h.
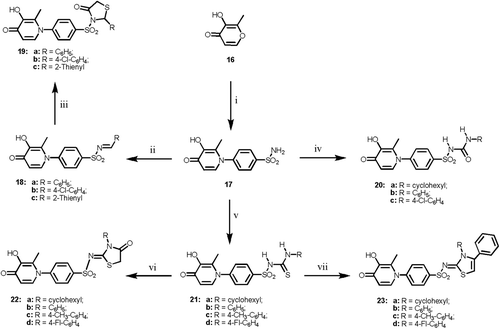
Table 1. Minimal inhibitory concentrations (MIC, μg/mL) of the active newly synthesized compounds.