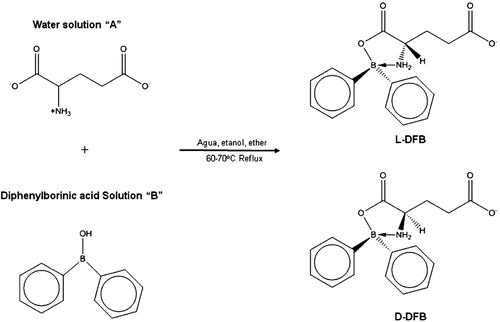
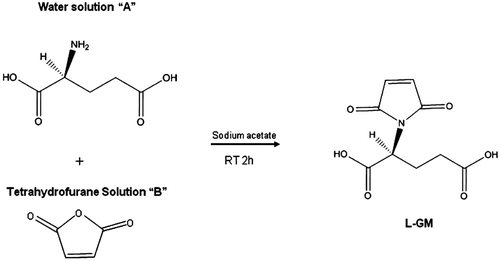
Figures & data
Figure 1. Binding site of: (a) d-glutamic acid and (b) l-glutamic acid on the RacE1 isoform with two principal amino acid residues, (c) d-glutamic acid and (d) l-glutamic acid on the RacE2 isoform with two principal amino acid residues. Carbon atoms are colored cyan; oxygen atoms, red; nitrogen atoms, blue; sulfur atoms of the protein, yellow for enzyme active site.
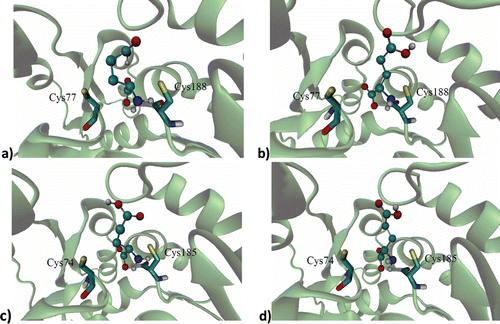
Figure 2. Binding site of: (a) l-GM, (b) d-DFB, (c) l-DFB on the RacE1 isoform, with two principal amino acid residues. Carbon atoms are colored cyan; oxygen atoms, red; nitrogen atoms, blue; sulfur atoms, yellow for enzyme active site.
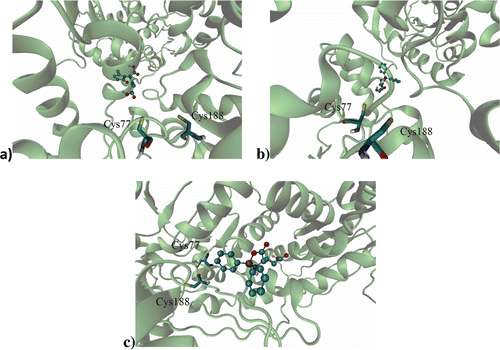
Figure 3. Ligand on racemase enzyme allosteric site of: (a) l-GM, (b) d-DFB, (c) l-DFB on the RacE1 isoform, with some amino acid residues. Carbon atoms are colored cyan; oxygen atoms, red; nitrogen atoms, blue.
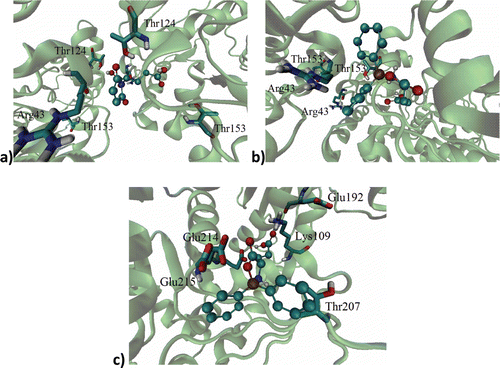
Table 1. Free energies values and Kd of the natural substrates of l and d-glutamic acid and all three test compounds in relation to RacE1 and RacE2, according to docking studies.
Table 2. MIC50 and MIC90 for the Bacillus spp. strains tested, by the microdilution technique in Mueller Hilton broth.
Table 3. Determination of IC50 of compound on Bacillus spp.