Figures & data
Figure 1. Structural motifs of AChE inhibitors BYYT-25, Donepezil, Acotiamide, and the synthesized compounds 5a–5p.
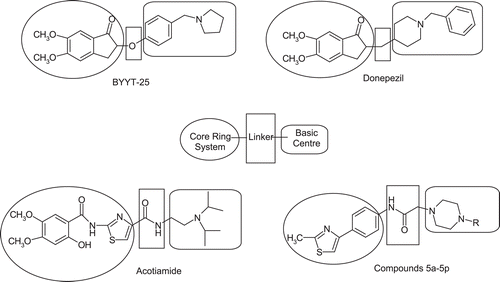
Table 1. Some physicochemical characteristics of the synthesized compounds.
Scheme 1. Synthesis of the compounds (5a–5p). Reagents: (i) acetyl chloride, TEA, THF, 0–5°C; (ii) Br2, AcOH; (iii) thioacetamide, EtOH, r.t.; (iv) 1 N HCl, EtOH, reflux; (v) chloroacetyl chloride, TEA, THF, r.t.; (vi) appropriate 4-substituted piperazine, K2CO3, acetone, reflux.
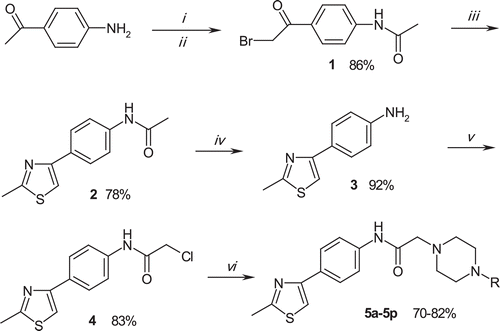
Table 2. Percentage AChE and BChE inhibition of the compounds and IC50 values.