Figures & data
Figure 1. Thermal stability expressed through the CD spectrum of rBm-33 heat treated to (95°C). Thermal stability of rBm-33 on pepsin inhibition activity.*The percentage activity was calculated assuming the maximum activity at 37°C is 100% (A). CD spectrum of heat treated (95°C) rBm-33 diluted with water at final concentration of 0.1 mg/ml at pH 6.9 (B).
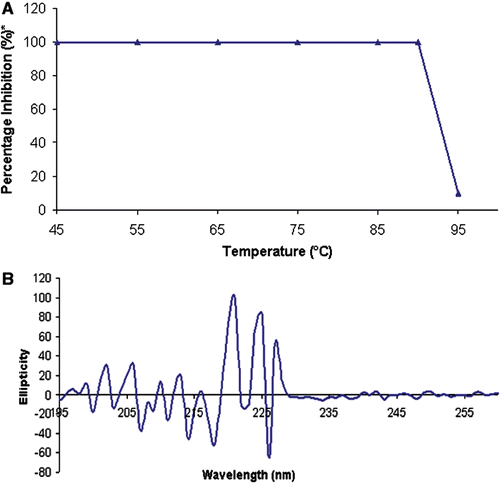
Figure 2. Immunoblot to show the removal of N-terminal his-tag from rBm-33 after the incubation with pepsin. M: protein molecular-weight marker; Lane 1: flow-through of IMAC (Ni2+-IDA) experiment containing glutaraldehyde cross linked rBm-33-pepsin complex probed with anti Bm-33 antisera; Lane 2: same flow-through probed with anti his-tag antibody.

Figure 3. Inhibition activity of rBm-33 against human aspartic proteases using UV spectroscopy. CA: only casein, P: pepsin, RE: renin, CE: cathepsin-E, CD: cathepsin-D, PS: pepstatin Bm: rBm-33. All the values represent the mean of six different experiments ± SD
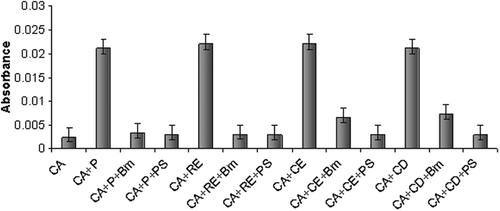
Figure 4. Kinetics of pepsin inhibition by rBm-33. The assay was performed with the fixed quantity of pepsin (5 mm) and varying concentrations of rBm-33 in absence and in the presence of (1 mM, 2.5 mM and 5 mM, respectively). Substrate-velocity curve (A) and Lineweaver-Burk plot (1/V vs. 1/S) (B) are shown indicating competitive inhibition of pepsin by rBm-33 (Ki = 2.5 (± 0.8) nM). P: pepsin (5 mM), P+ [B 1 mM]: pepsin+rBm-33 (1 mM), P+ [B 2.5 mM]: pepsin+rBm-33 (2.5 mM), P+ [B 5 mM]: pepsin+rBm-33 (5 mM). Assay was carried out in triplicates and the kinetic constants were determined using Graphpad Prism 2.0 (San Diego, CA, USA).
![Figure 4. Kinetics of pepsin inhibition by rBm-33. The assay was performed with the fixed quantity of pepsin (5 mm) and varying concentrations of rBm-33 in absence and in the presence of (1 mM, 2.5 mM and 5 mM, respectively). Substrate-velocity curve (A) and Lineweaver-Burk plot (1/V vs. 1/S) (B) are shown indicating competitive inhibition of pepsin by rBm-33 (Ki = 2.5 (± 0.8) nM). P: pepsin (5 mM), P+ [B 1 mM]: pepsin+rBm-33 (1 mM), P+ [B 2.5 mM]: pepsin+rBm-33 (2.5 mM), P+ [B 5 mM]: pepsin+rBm-33 (5 mM). Assay was carried out in triplicates and the kinetic constants were determined using Graphpad Prism 2.0 (San Diego, CA, USA).](/cms/asset/33d26180-ae45-4d04-9cdf-15c0a90d0f38/ienz_a_710849_f0004_b.gif)
Table 1. Association constants (Kb) and thermodynamic parameters for the binding of important human aspartic proteases to rBm-33.
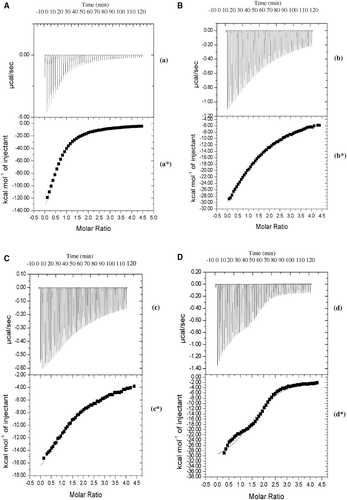
Figure 5. Iso-thermal calorimetric titration of rBm-33 (refolded) with cathepsin-E (C). Raw data obtained from 50 automatic injections of 5 µl aliquots of 0.036 mM cathepsin-E into 0.036 mM of rBm-33 (refolded) at 298 K (c). Non-linear least squares fit for the cathepsin-E and rBm-33 (refolded) (c*). Iso-thermal calorimetric titration of rBm-33 (refolded) with cathepsin-D (D). Raw data obtained from 50 automatic injections of 5 µl aliquots of 0.036 mM cathepsin-D into 0.036 mM of rBm-33 (refolded) at 298 K (d). Non-linear least squares fit for the cathepsin-D and rBm-33 (refolded) (d*).