Figures & data
Figure 1. The basic adenylate cyclase activity was evaluated in the presence of the increasing concentrations of the monovalent salts choline-Cl, NaCl, KCl and RbCl (1 mM MgCl2; 1 mM ATP). The effect of the increased monovalent ions on the basic adenylate cyclase activity, expressed statistically significant inhibitory effect on the activity of the adenylate cyclase in the concentrations range of the used monovalent salts from 30 to 200 mM. Full dark circle represents Choline-Cl, full white circle represents NaCl, full dark square represents KCl and full white square represents RbCl.
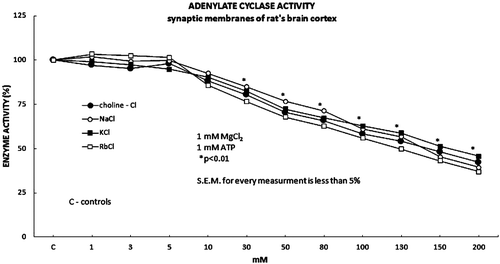
Figure 2. The inhibitory part of the kinetic curve for adenylate cyclase activity is presented in the form of Dixon’s graph. In the presence of the increasing concentrations of the RbCl (1 mM MgCl2, 1 mM ATP), the kinetic activity value Ki for the adenylate cyclase was 27 Mm.
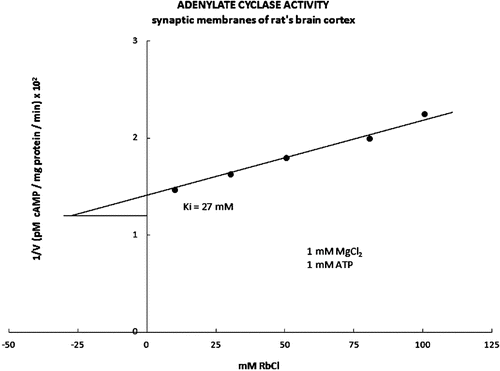
Figure 3. The adenylate cyclase activity was stimulated with 5 μM GTPτS in the presence of 1 mM MgCl2 and the monovalent ions in forms of their inorganic salts, choline-Cl, NaCl, KCl and RbCl. All tested monovalent ions exhibited significant inhibitory effect on the adenylate cyclase activity when applied in the higher concentrations (50 mM) compared to the control (30 mM). The investigated monovalent ions expressed both the statistically significant and the permanent inhibitory effect on the adenylate cyclase activity in the concentration range from 50 to 200 mM. Full dark circle represents Choline-cl, full white circle represents NaCl, full dark square represents KCl and full white square represents RbCl.
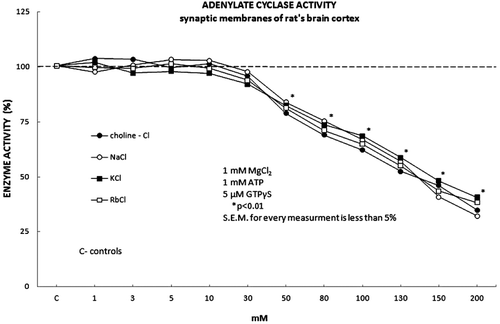
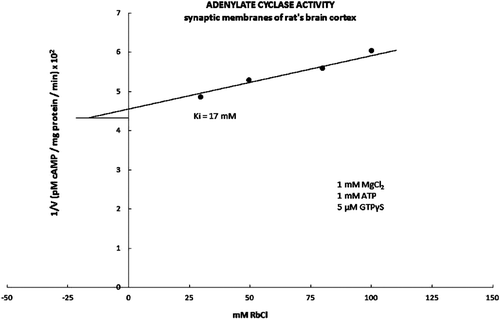
Figure 4. The inhibitory part of the kinetic curve for the adenylate cyclase activity is presented in the form of Dixon’s graph. The adenylate cyclase activity was stimulated with 5 μM GTPτS in the presence of the increasing concentrations of the RbCl. The kinetic activity value Ki for the adenylate cyclase was 17 mM, which is statistically significantly lower compared to the controlled value Ki for the basic kinetic activity of the adenylate cyclase (Ki = 27 mM).