Figures & data
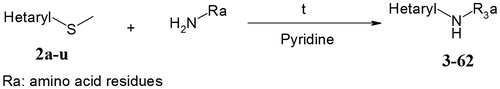
Table 1. Chemical structures of building-blocks 2a–2u.
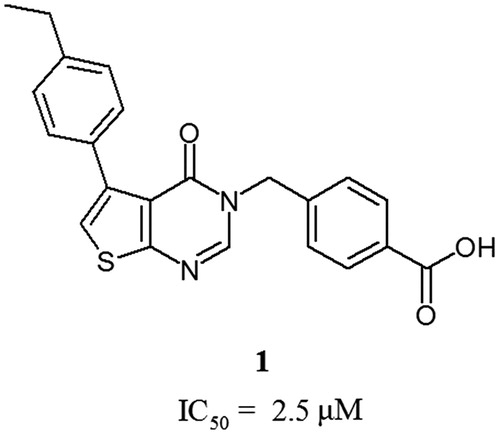
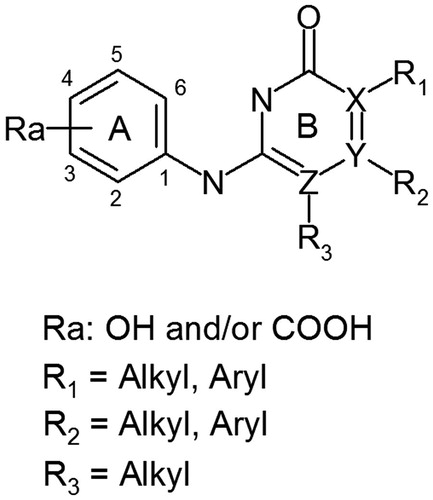
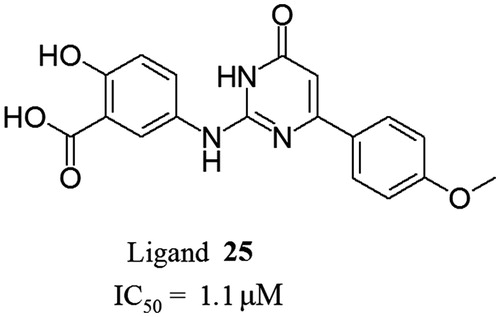
Table 2. Chemical structures and in vitro activities of compounds 3–15.
Table 3. Chemical structures and in vitro activities of compounds 16–34.
Table 4. Chemical structures and in vitro activities of compounds 35–62.
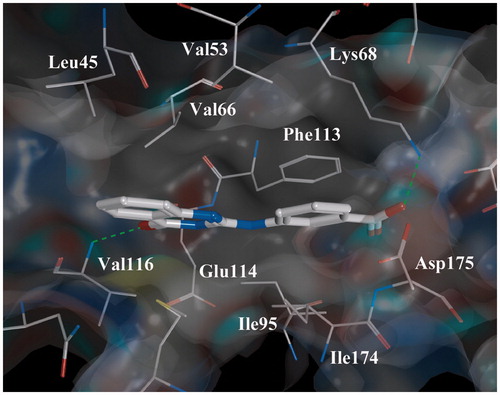
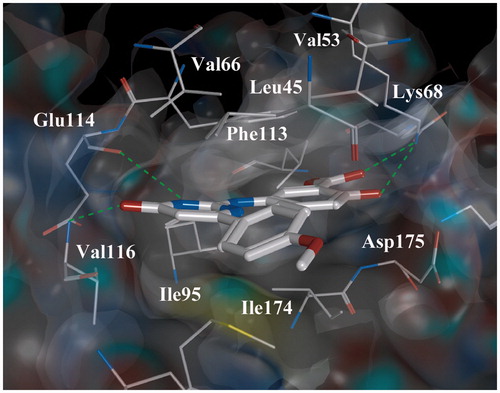
Figure 3. Complex “compound 7-CK2” obtained by the molecular docking method. Intermolecular H-bonds are shown as dotted lines.