Figures & data
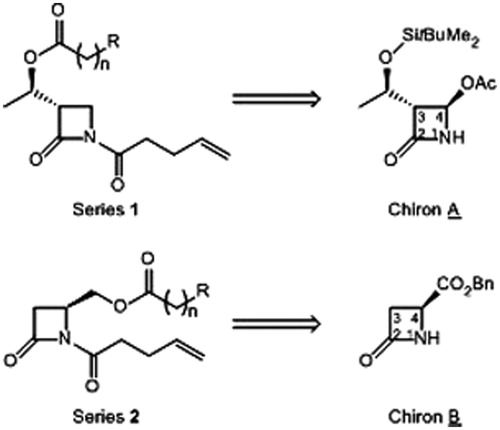
Scheme 1. Synthesis of 1,4-disubstituted β-lactams: (i) NaBH4, MeOH, 0–20 °C, 3 h; (ii) carboxilic acid, DCC, DMAP (catal.), DCM, 20 °C, 24 h; (iii) acid chloride, pyridine or DMAP, DCM, 20 °C, 24 h; (iv) ClO-(CH2)2-CH=CH2, pyridine, DCM, reflux, 24 h; (v) ClCO-(CH2)2-CH=CH2, LiHMDS, THF-DMF, −78–20 °C, 1 h. See for yields of 4 and 2.
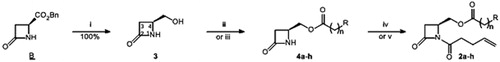
Table 1. Yields of compounds (%) of the .
Table 2. hFAAH and hMAGL inhibition.
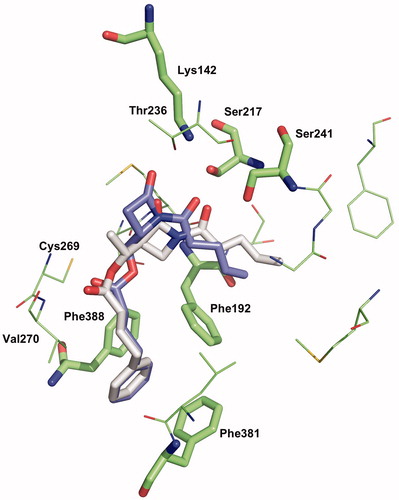
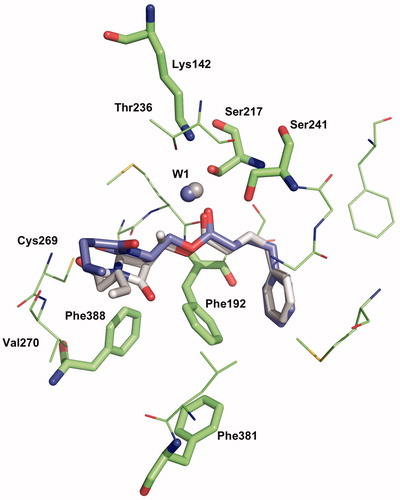
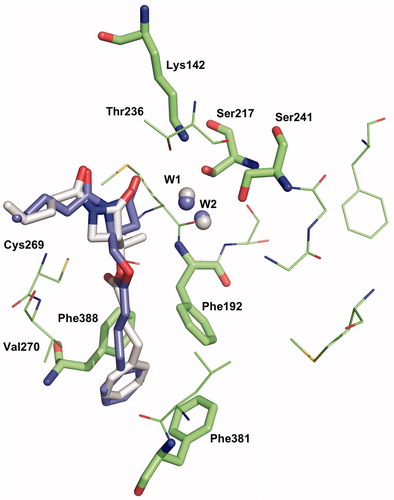
Figure 6. Binding mode 1 of 1a and 2a in the active site of hFAAH. Hydrogens were removed for clarity.