Figures & data
Scheme 1. Synthesis of 7H-dibenzo[de,g]quinolin-7-one 4. Reagents and conditions: (1) DMAD/toluene/reflux/8 days; (2) KOH/CH3OH/H2O (85 °C); (3) Diphenyl ether (250 °C).
![Scheme 1. Synthesis of 7H-dibenzo[de,g]quinolin-7-one 4. Reagents and conditions: (1) DMAD/toluene/reflux/8 days; (2) KOH/CH3OH/H2O (85 °C); (3) Diphenyl ether (250 °C).](/cms/asset/0f7bc57f-3ecb-49cf-a99a-286cff0963c0/ienz_a_845818_f0002_b.jpg)
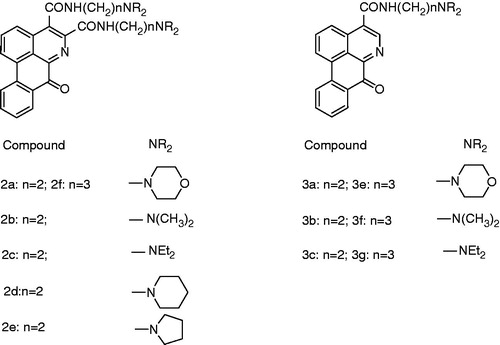
Figure 3. Synthesis of disubstituted derivatives. Reagents and conditions: (1) SOCl2/C6H6; (2) NH2 (CH2)nNR2/Et3N.
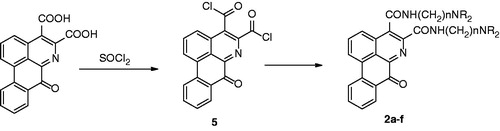
Figure 4 Inhibition of Topo I-catalyzed DNA relaxation by 7-oxoaporphine and Camptothecin. Lane 1, pBR322 DNA only. Lane 2, pBR322 DNA and Topo I. Lanes 3–7, pBR322 DNA, Topo I, and various drug concentrations.
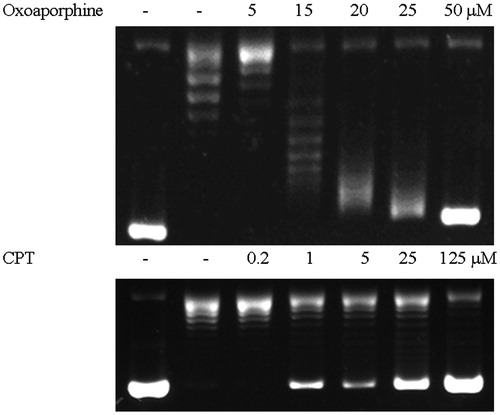
Figure 5 Absorption titration of 7-oxoaporphine with CT DNA. Compound 7-oxoaporphine (20 µM) in 10 mM sodium phosphate buffer (pH 7.0) with 150 mM NaCl at increasing CT DNA concentration (arrow: 0–400 µM).
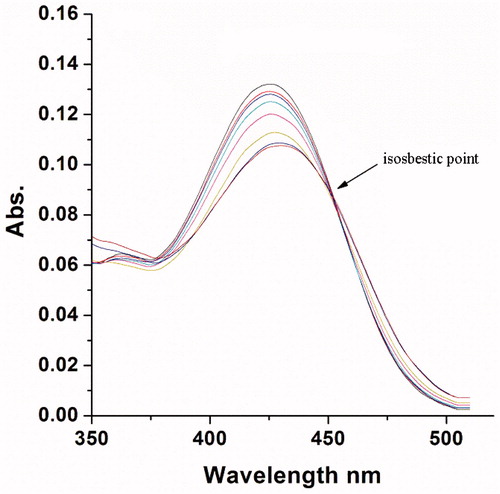
Table 1. Binding constants (Ki) and photometric properties of oxoaporphine derivatives in contact with CT DNA.
Table 2. IC50 cytotoxicity values (µM) of oxoaporphine derivatives against tumor cells.