Figures & data
Figure 1. HPLC analysis of the red pigment fraction after silica gel column chromatography. Pigments were subjected to HPLC using an isocratic mobile phase of 55% aqueous methanol solution containing 0.2% acetic acid. Seven pigments are indicated as P-1 to P-7 according to the order of elution.
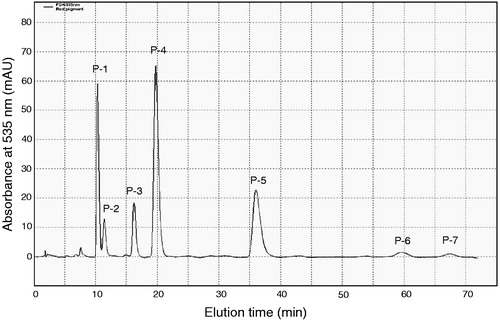
Figure 2. Chemical structures of prodigiosin congeners: n = 3, 2-methyl-3-butylprodiginine (P-2); n = 4, 2-methyl-3-pentylprodiginine (prodigiosin) (P-4); n = 5, 2-methyl-3-hexylprodiginine (P-5); n = 6, 2-methyl-3-heptylprodiginine (P-6).
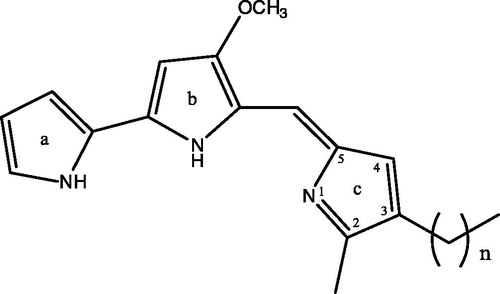
Figure 3. Effects of the prodigiosin congeners on the U937 human leukemia cell line. U937 cells were incubated for 24 h with each prodigiosin congener at a concentration of 2 μM. Cell viability was evaluated in duplicate by the MTS assay.
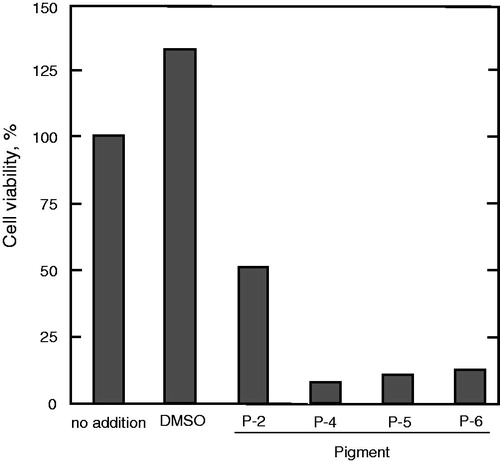
Figure 4. Effect of the prodigiosin compounds on the activity of CaM kinase. The CaM kinase assay was conducted in the presence of the indicated concentrations of the mixture of prodigiosin compounds. The values of enzyme activity assayed in duplicate were within 10% of each other and the mean values are shown. Enzyme activity at 100% (no addition) is 0.611 nmol Pi incorporated/20 min.
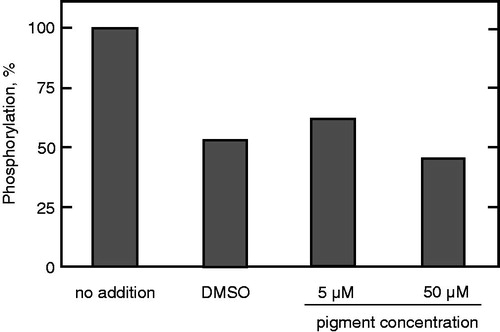
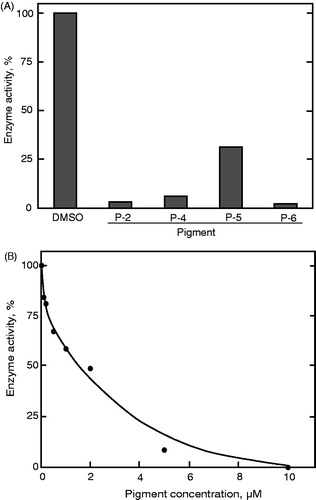
Figure 5. Inhibition of PTP1B by the prodigiosin compounds. The assay was carried out in the presence of (A) the mixture of the compounds at different concentrations and (B) individual prodigiosin compounds at 10 μM. Data represent a typical result from two independent experiments and the mean values of enzyme activity assayed in duplicate. Values in duplicate assay were within 10% of each other.
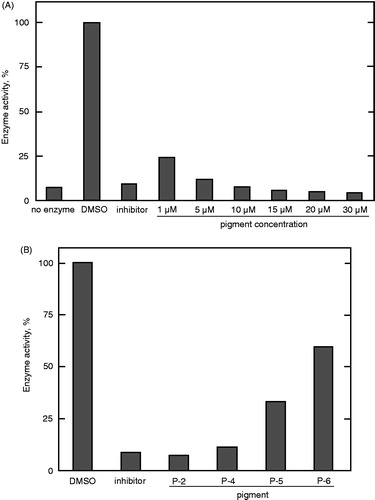
Figure 6. Inhibition of PP2A activity by the prodigiosin congeners. (A) The assay was conducted in the presence of the individual compounds at 10 μM. (B) Enzyme inhibition was assayed in the presence of P-2 at different concentrations. Data represent a typical result from two independent experiments and the mean values of enzyme activity assayed in duplicate. Values in duplicate assay were within 10% of each other.