Figures & data
Figure 1. Pymol generated view of the uterotonic polypeptide kalata B1structure characterized by three disulphide bonds.
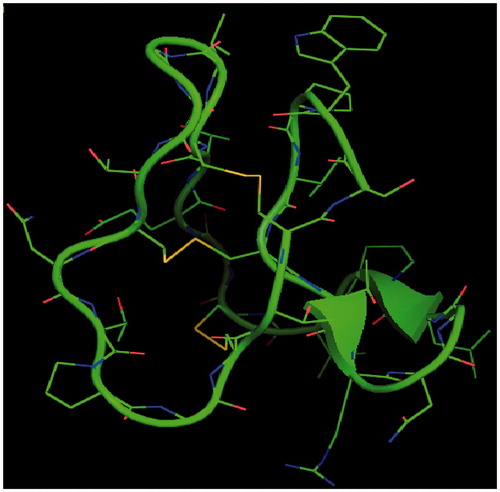
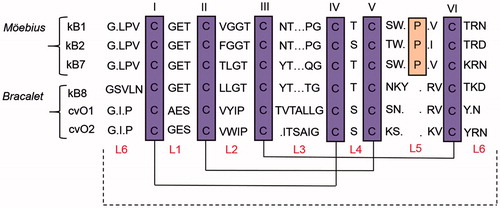
Figure 2. Sequences of significative cyclotides belong to the two subfamilies Möebius type and Bracelet type.
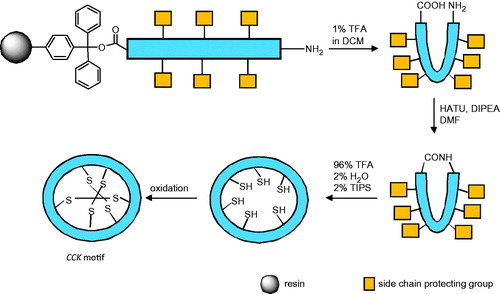
Scheme 1. Schematic representation of the double use of cyclotides; on the right: natural sources of bioactive compounds; on the left: scaffold engineered bearing an external bioactive peptide sequence.
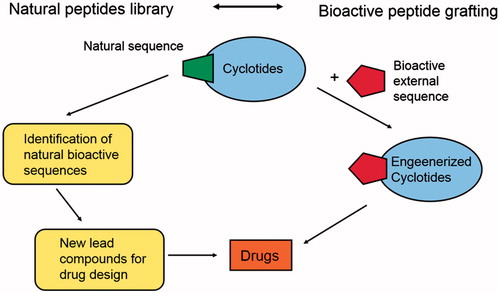
Table 1. Kalata B7 and loop 3 fragment analogues with oxytocic and vasopressin like activityCitation20.
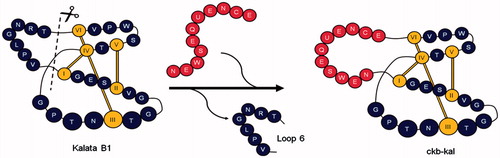
Figure 4. Bioactive peptide (new sequence) grafting in place of the native loop 6 of cyclotide scaffold.