Figures & data
Figure 1. Amino acid sequence alignment of the γ-CAs from Porphyromonas gingivalis (PgiCA), Vibrio cholerae (VhCA) and Brucella suis (bSuCA). The metal ion ligands are indicated in red. The multialignment was performed with the program Clustal W. The asterisk (*) indicates identity at all aligned positions; the symbol (:) relates to conserved substitutions, while (.) means that semi-conserved substitutions are observed.

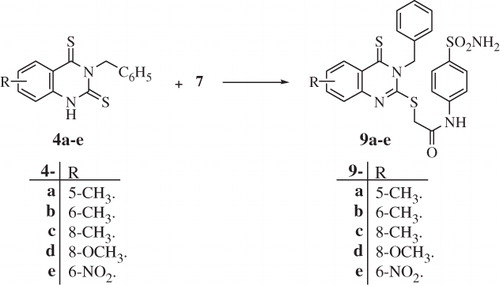
Figure 2. Phylogenetic trees of the amino acid sequences of γ-CAs from different Gram-negative bacteria. The tree was constructed using the program PhyML 3.0. Legend: EcoCA, Escherichia coli; VchCA, Vibrio cholerae; SspCA, Sulfurihydrogenibium yellowstonense; SazCA, Sulfurihydrogenibium azorense; PgiCA, Porphyromonas gingivalis; bSuCA, Brucella suis; BpsCA, Burkholderia pseudomallei; ReuCA, Ralstonia eutropha.
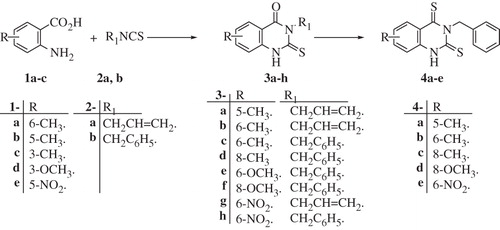
Scheme 1. Synthesis of 3,5,6 and/or 8-substituted-2-thio-4-oxoquinazoline and 2-thio-4-thioxoquinazoline derivatives 3a–h and 4a–e.
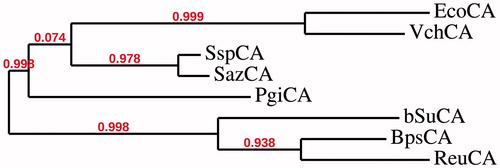
Scheme 2. Synthesis of substituted sulfa derivative 7 and 2,3,5,6 and/or 8-substituted-4-oxoquinazoline derivatives 8a–h.