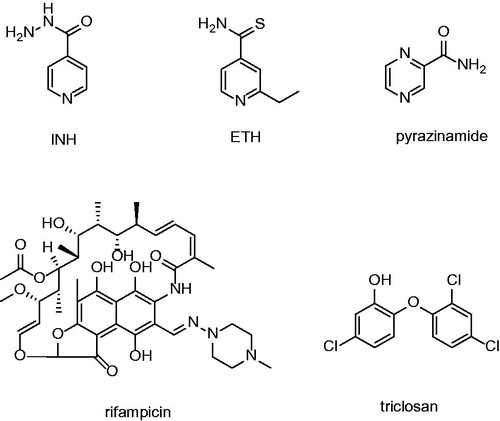
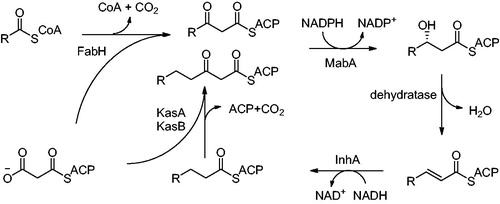
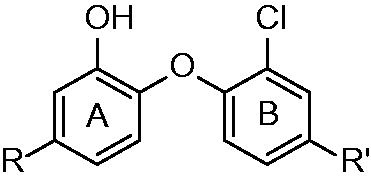
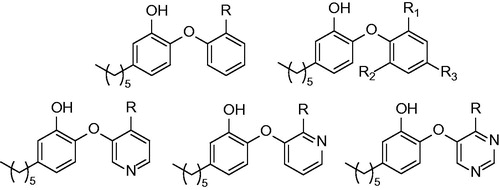
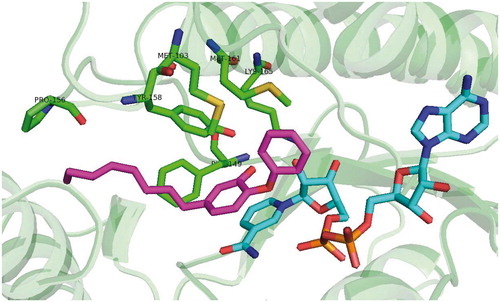
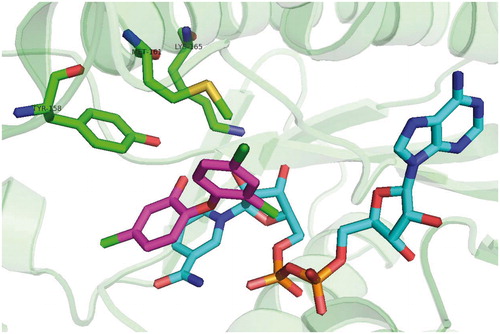
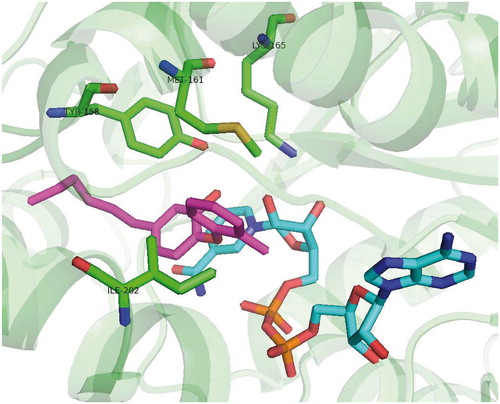
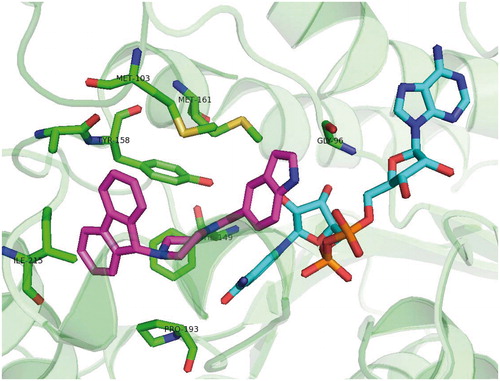
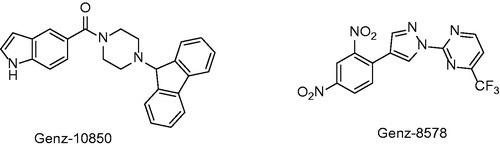
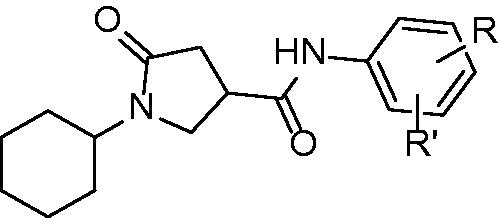
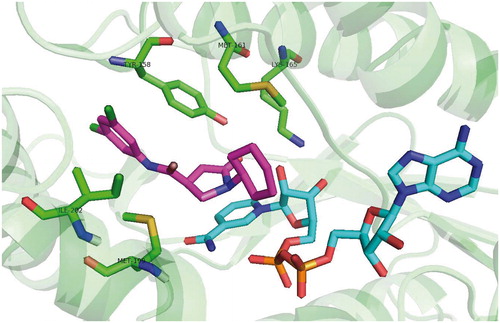
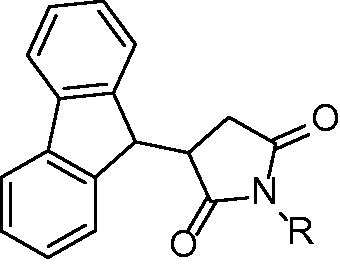
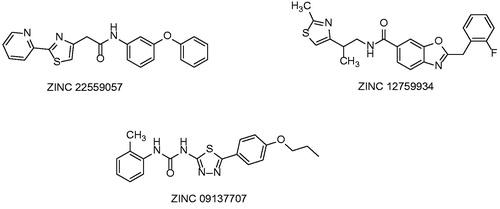
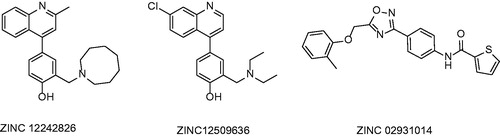
Figures & data
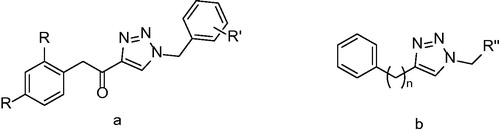
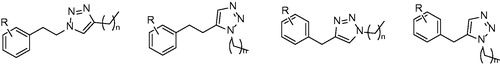
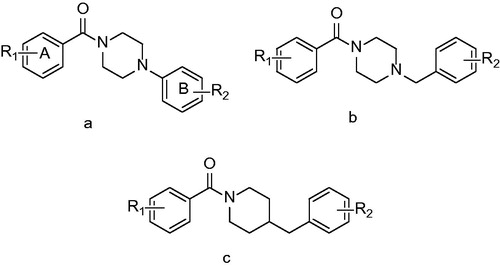
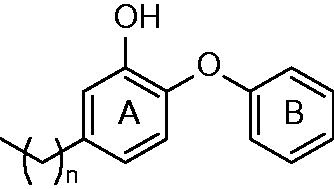
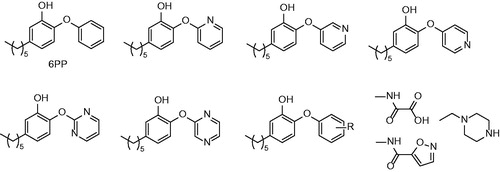
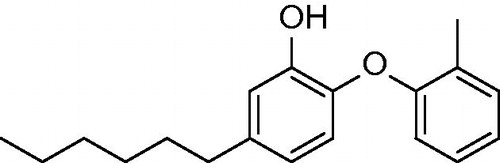
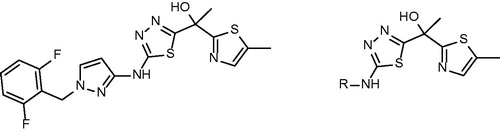
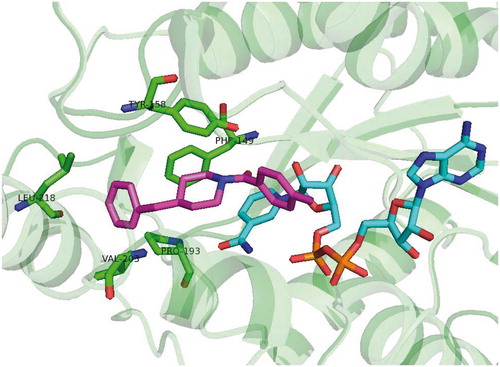
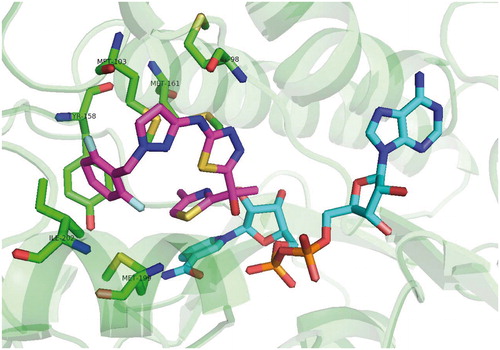
Table 1. Structures and in vitro results of notable InhA inhibitors.