Figures & data
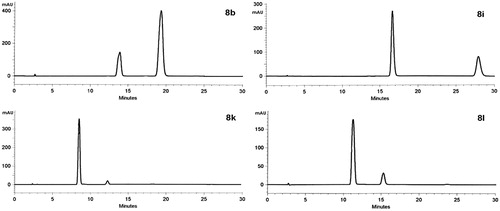
Figure 2. Normal-phase HPLC diastereomeric resolution of compounds 8b, 8i, 8k and 8l. Column: Kromasil 100-5SIL microporous silica column (25 cm × 4.6 mm); eluent: hexane–ethyl acetate (80:20); flow rate:1.2 mL/min; detection: 304 nm.
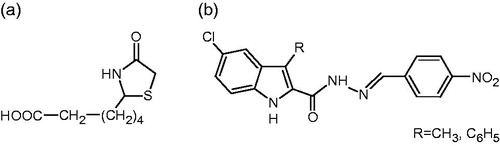
Scheme 1. Synthesis of 6–8. Reagents and conditions: (i) 7% NaNO2, EtOH, conc. HCl, 0 °C; (ii) ethyl 2-benzyl-3-oxo-butanoate, KOH, EtOH, 0 °C; (iii) conc. HCl, reflux, 4 h; (iv) H2NNH2·H2O, EtOH, reflux, 6 h; (v) (non)substituted benzaldehyde, abs. EtOH, reflux, 5–6 h; (vi) mercaptoacetic acid, dry benzene, reflux, 5–6 h and (vii) 2-mercaptopropionic acid, dry benzene, reflux, 5–6 h.