Figures & data
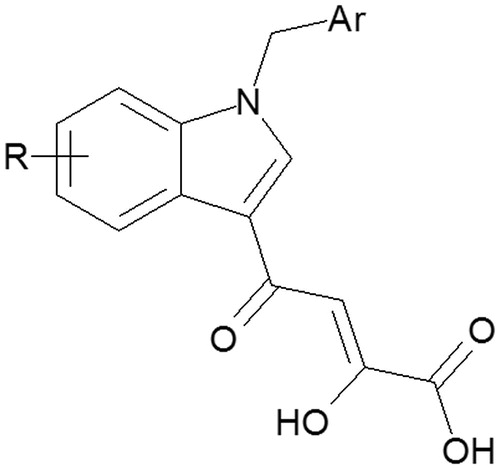
Scheme 1. Reagents and conditions: (i) POCl3, CH3CON(CH3)2, RT, 12 h; (ii) benzyl bromide or chloride, NaH, DMF, mw: 10 min at continuous temperature (50 °C), 100 W; (iii) diethyl oxalate, dry CH3ONa, THF, two separate steps under the same conditions mw: 2 min, at continuous temperature (50 °C), 250 W and (iv) 2 N NaOH, MeOH, RT, 1.5 h.
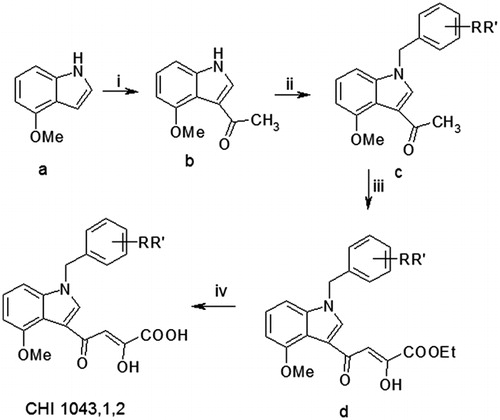
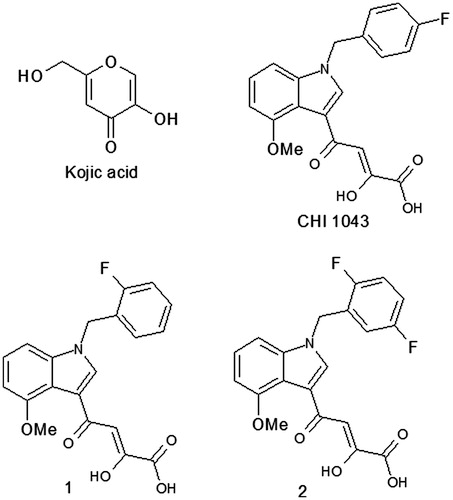
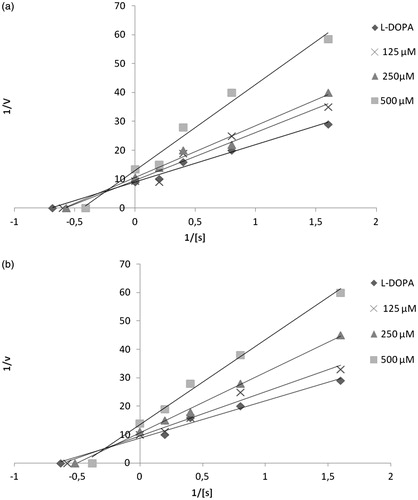
Table 1. Inhibitory activity against mushroom tyrosinase of indole derivatives.
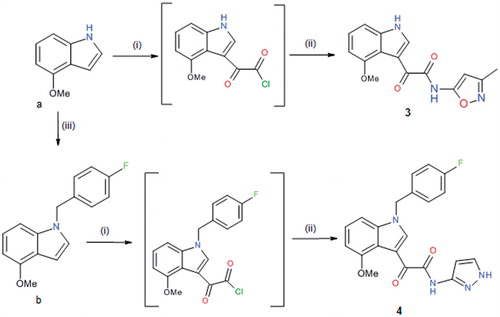
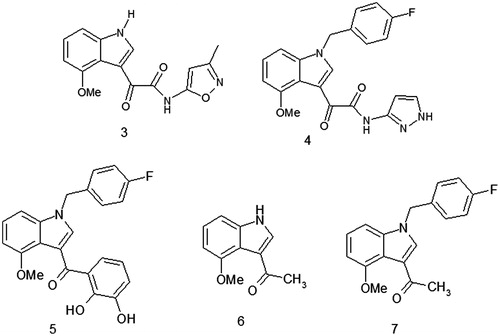
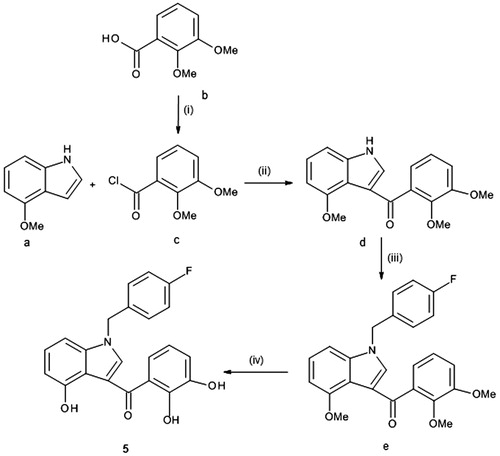
Scheme 2. Reagents and conditions: (i) oxalyl chloride, dry Et2O, N2, 0 °C, 3 h; (ii) NH2-Het, dry THF, N2, rt, 1.5 h and (iii) 4-fluorobenzyl bromide, t-BuOK, THF, rt, 2 h.