Figures & data
Figure 2. X-ray molecular structure of compound 7a with thermal ellipsoids at 50% probability level.
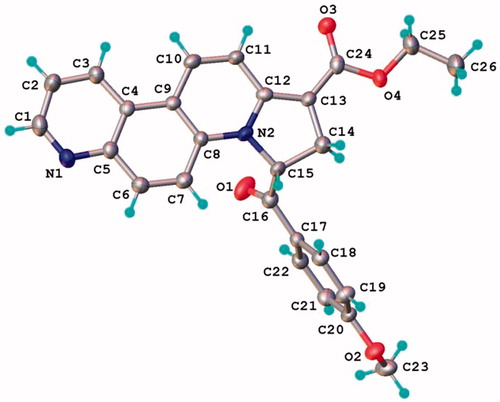
Figure 3. View of supramolecular columnar architecture in the crystal structure of 7a. Only H atoms involved in hydrogen bonding are shown. H-bonds parameters: C10–H⋯O3 [C10–H 0.93 Å, H⋯O3 2.38 Å, C10⋯O3(2 − x, 1 − y, −z) 3.308(4) Å, C10–H⋯O3 175.8 °]; C3–H⋯O3 [C3–H 0.93 Å, H⋯O3 2.49 Å, C3⋯O3(2 − x, 1 − y, −z) 3.419(4) Å, C10–H⋯O3 173.0°]; C14–H⋯O3 [C14–H 0.97 Å, H⋯O3 2.44 Å, C14⋯O3(−1 + x, y, z) 3.299(4) Å, C10–H⋯O3 148.0°].
![Figure 3. View of supramolecular columnar architecture in the crystal structure of 7a. Only H atoms involved in hydrogen bonding are shown. H-bonds parameters: C10–H⋯O3 [C10–H 0.93 Å, H⋯O3 2.38 Å, C10⋯O3(2 − x, 1 − y, −z) 3.308(4) Å, C10–H⋯O3 175.8 °]; C3–H⋯O3 [C3–H 0.93 Å, H⋯O3 2.49 Å, C3⋯O3(2 − x, 1 − y, −z) 3.419(4) Å, C10–H⋯O3 173.0°]; C14–H⋯O3 [C14–H 0.97 Å, H⋯O3 2.44 Å, C14⋯O3(−1 + x, y, z) 3.299(4) Å, C10–H⋯O3 148.0°].](/cms/asset/218d1528-e6f5-437c-8737-207ad727215f/ienz_a_1039530_f0003_c.jpg)
Figure 4. The X-ray molecular structure of compound 11f with the thermal ellipsoids at 50% probability level.
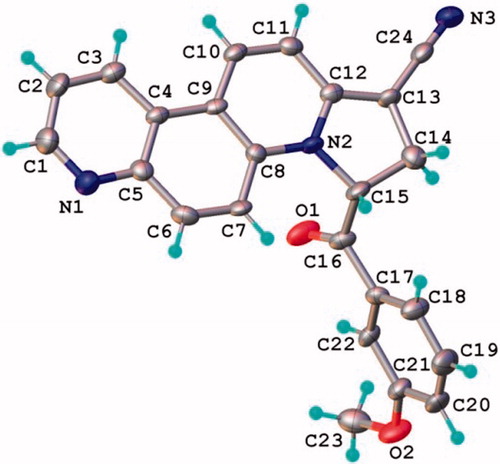
Figure 5. View of supramolecular columnar architecture in the crystal structure of 11f. Only H atoms involved in hydrogen bonding are shown. H-bonds parameters: C7–H⋯O1 [C7–H 0.93 Å, H…O1 2.48 Å, C7⋯O1(1 + x, y, z) 3.158(5) Å, C10–H⋯O3 129.8 °].
![Figure 5. View of supramolecular columnar architecture in the crystal structure of 11f. Only H atoms involved in hydrogen bonding are shown. H-bonds parameters: C7–H⋯O1 [C7–H 0.93 Å, H…O1 2.48 Å, C7⋯O1(1 + x, y, z) 3.158(5) Å, C10–H⋯O3 129.8 °].](/cms/asset/10234eb5-549d-46c0-adfe-8067ee6951e7/ienz_a_1039530_f0005_c.jpg)


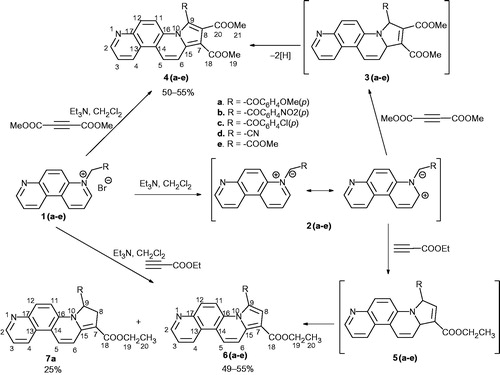
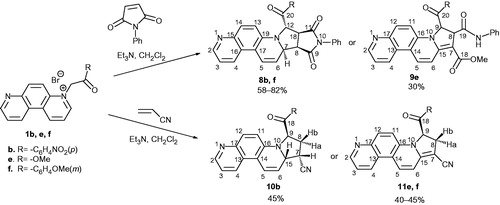
![Scheme 3. Design in the class of pyrrolo[2,1-c][4,7]phenanthroline derivatives.](/cms/asset/d518a9d2-430f-4ca0-8294-8e984b346505/ienz_a_1039530_sch0003_c.jpg)