Figures & data
Figure 1. Three main groups of azole NNRTIs: aryl–CH2–(–NH–, –O–) disubstituted azoles (a), azole-thioglycolanilide derivatives (b) and other (c).
Table 1. Considered R1, R2 and R3 substituents for triazole NNRTIs and selected results for Glide SP protocol in 2RKI receptor.
Table 2. Inhibitory activity (in µM) of triazoles 1–9 against HIV-1 RT (wild type, Innovagen kit).
Table 3. Structures of the 5-position substitution variants used for docking experiments and selected results for Glide SP protocol in 2RKI receptor.
Scheme 1. General synthetic route and structures of examined compoundsa. R1, R2 and R3 substituents are presented in and . aReagents and conditions: (a) diethyl ether, 48 h, rt. (b) 2% NaOH, reflux, 2h. (c) 3M HCl, rt. (d) BrCH2COBr, K2CO3, acetonitrile, 0 °C. (e) K2CO3, KI, methanol, 1–2 h, rt. (f) PhCH2Br, K2CO3, KI, methanol, 1–2 h, rt.
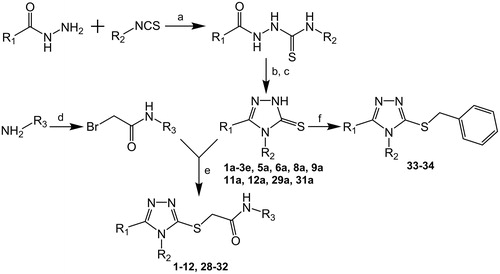
Table 4. Inhibitory activity (in µM) of triazoles 10–12, 28–34 against HIV-1 RT (wild type, Roche kit).
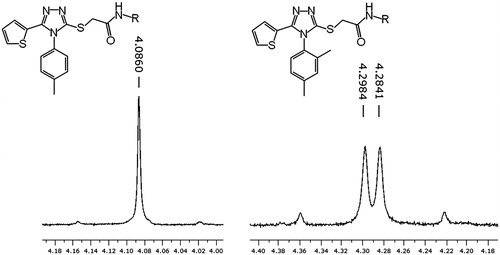
Figure 2. 1H-NMR spectra (250 MHz) showing the splitting of the signal of methylene protons resulting from constraining the phenyl group rotation by the 2-methyl substituent.