Figures & data
Figure 2. Binding sites A and B and designation of the torsion angles θ1 and θ2 of the compounds synthesized.
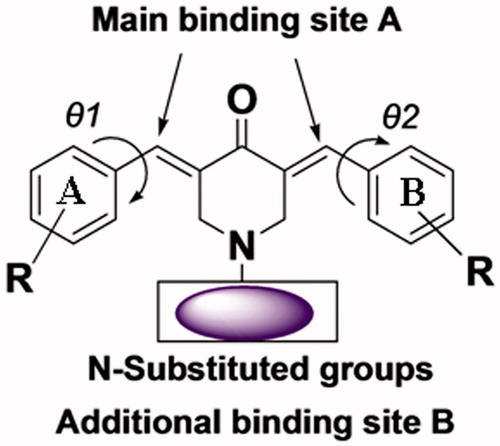
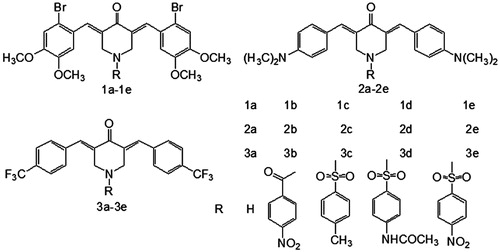
Scheme 1 Synthetic chemical pathway of compounds in series 1–3. The reagents used in the syntheses were as follows: (I) 2-bromo-4,5-dimethoxy-benzaldehyde/CH3COOH/dry HCl; (II) 4-dimethylamino-benzaldehyde/CH3COOH/dry HCl; (III) 4-trifluoromethyl-benzaldehyde/CH3COOH/dry HCl; (IV) 4-nitrobenzoyl chloride/NaOH/ClCH2CH2Cl/K2CO3/tetrabutylammonium bromide or 4-methylbenzene sulfonyl chloride/4-acetamidobenzenesulfonyl chloride/4-nitrobenzenesulfonyl/NaOH/ClCH2CH2Cl/tetrabutylammonium bromide/CH3COOH.
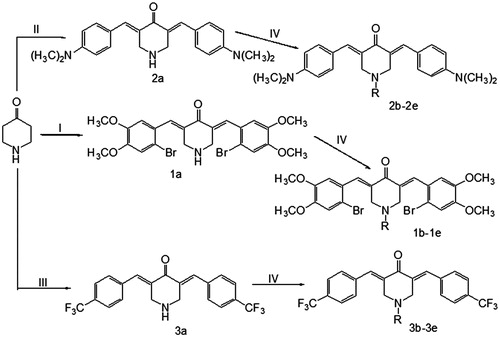
Table 1. Cytotoxicity of 1a–1e, 2a–2e and 3a–3e against human SK-BR-3, PG-BE1, NCI-H460, MIA PaCa-2 and SW1990 cell lines.
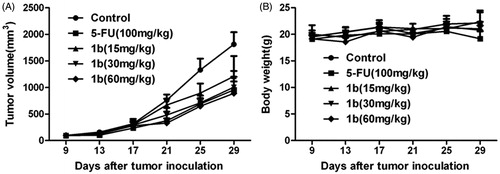
Figure 3. The inhibitory effect of compounds 1a–1e and 3a–3e against five human carcinoma cell lines.
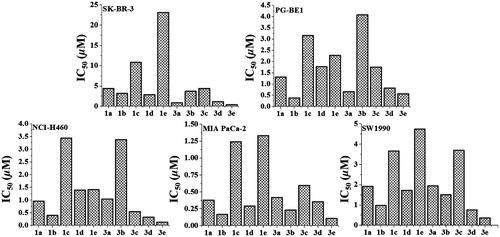
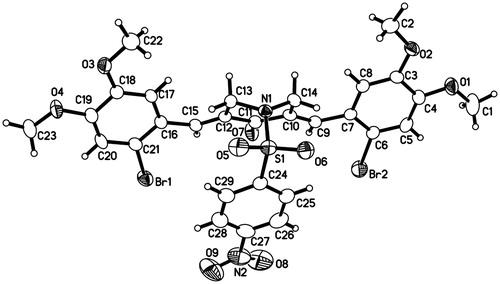
Figure 4. The ORTEP figure of 1e (displacement ellipsoids with 30% probability, omitting two molecules of CHCl3).