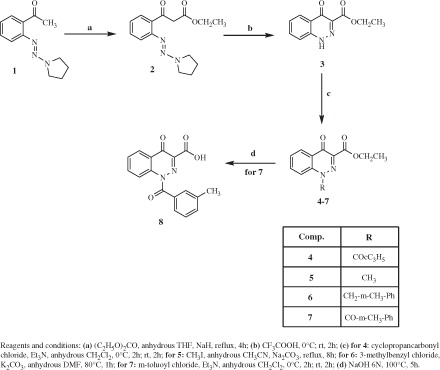
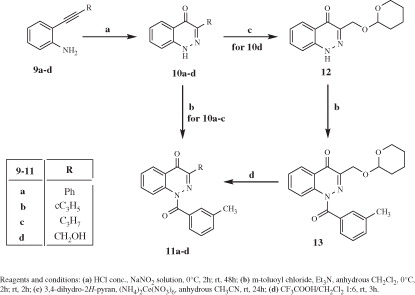
Figures & data
Table 1. HNE inhibitory activity of cinnolone derivatives 4–8, 11a–d, 16a–c, and 18a–g.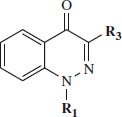
Table 2. HNE Inhibitory activity of cinnolone derivatives 21a–d and 24.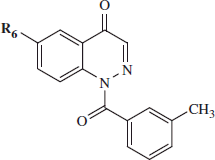
Table 3. HNE inhibitory activity of cinnoline derivatives 17a–c, 22, and 19.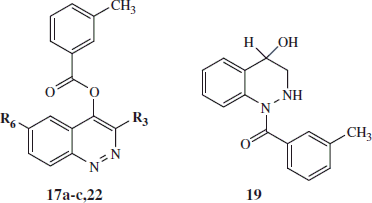
Figure 2. Kinetics of HNE inhibition by cinnoline derivative 18a. Representative double-reciprocal Lineweaver–Burk plot of substrate hydrolysis by HNE in the absence and presence of the compounds 18a is shown. The representative plot is from three independent experiments.
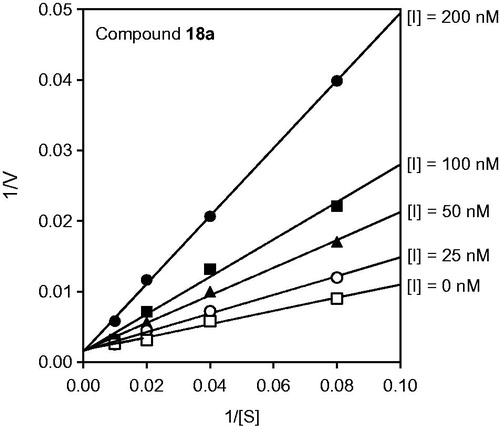
Table 4. Half-life (t1/2) for the spontaneous hydrolysis of selected cinnolinone derivatives.
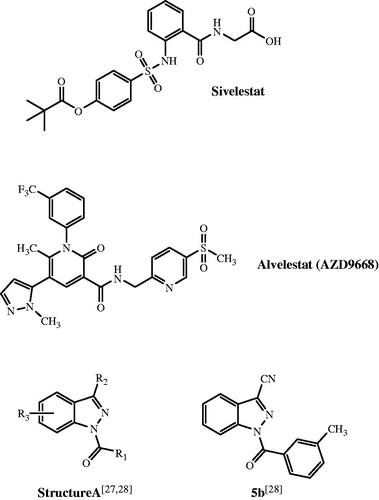
Figure 3. Superimposed docking poses of peptide chloromethyl ketone and novel small-molecule HNE inhibitors. Co-crystallized peptide chloromethyl ketone inhibitor is shown in yellow. Residues within 5 Å of this ligand are visible. Panel (A) Docking poses of reference compound 5bCitation28 (dark-green) and compound 18a (blue). Panel (B) Docking poses of compounds 16b (violet) and 17b (brown).
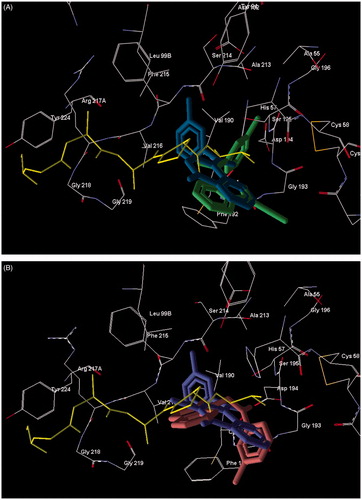
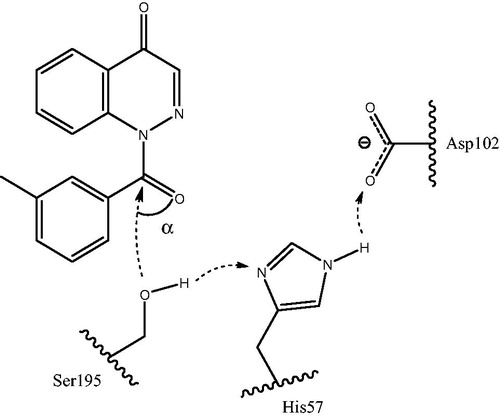
Figure 4. Hypothetical model for the nucleophilic attack of Ser195 at the carbonyl group of cinnoline derivative (18a) accompanied by synchronous proton transfer from Ser195 to Asp102 via the catalytic triad. The key angle α is indicated (see text for details). The model is based on the proposed mechanism of synchronous proton transfer from the oxyanion hole in serine proteasesCitation40,Citation47.