Figures & data
Figure 1. Total antioxidant activity of taxifolin and standards like trolox, α-tocopherol, BHT, and BHA at the same concentration (30 μg/mL) assayed by the ferric thiocyanate method in the linoleic acid system. The control value reached a maximum 60 h (BHA, butylated hydroxyanisole; BHT, butylated hydroxytoluene).
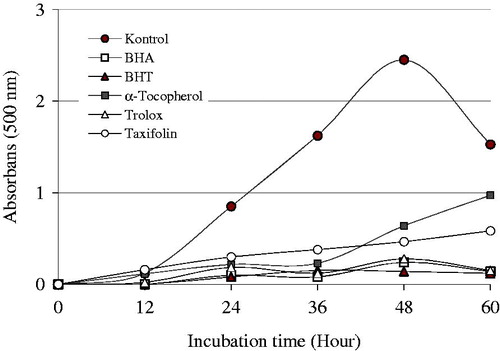
Table 1. Determination of half maximal concentrations (IC50) of taxifolin and standards belonging to Fe2+ chelating, DPPH√, ABTS√+, DMPD√+, and  scavenging assays.
scavenging assays.
Figure 2. Reducing power of taxifolin. (A) Fe3+→Fe2+ reductive potential of different concentrations (10–30 μg/mL) of taxifolin (r2: 0.960) and reference antioxidants. (B) Cu2+-reducing ability of different concentrations (10–30 μg/mL) of taxifolin (r2: 0.956) and reference antioxidants. (C) TPTZ-Fe3+→TPTZ-Fe2+ reductive potential of different concentrations (10–30 μg/mL) of taxifolin (r2: 0.993) and reference antioxidants (BHA, butylated hydroxyanisole; BHT, butylated hydroxytoluene).
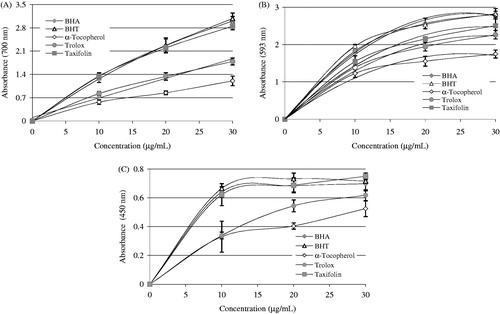
Figure 3. Comparison of Fe2+-chelating activity of taxifolin (r2: 0.942) and standards like trolox, EDTA, α-tocopherol, BHT, and BHA at the concentrations of 10–20 mg/mL (BHA, butylated hydroxyanisole; BHT, butylated hydroxytoluene).
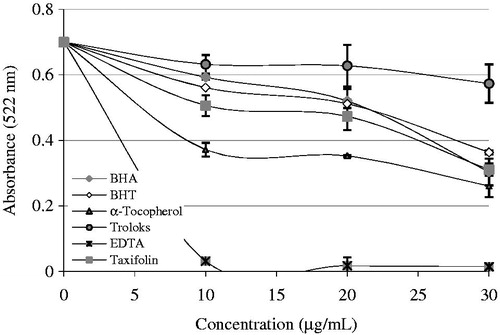
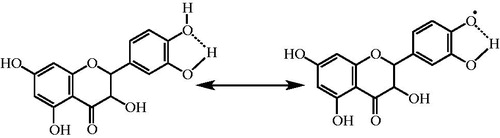
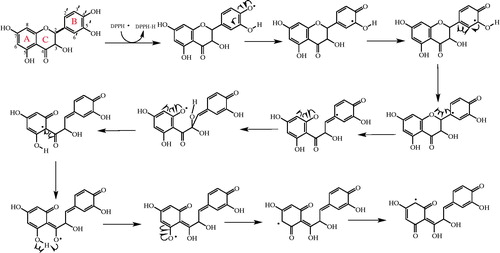
Figure 4. Possible places on taxifolin for chelating the transition metal ions such as Fe2+ in the process of lipid peroxidation.
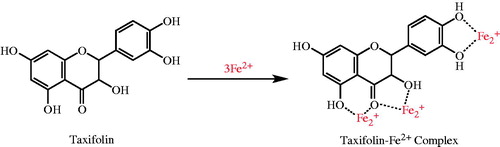
Table 2. Determination of reducing power of taxifolin by K3[Fe(CN)6] reduction and FRAP methods, cupric ions (Cu2+) reduction capacity by Cuprac and FRAP methods*.
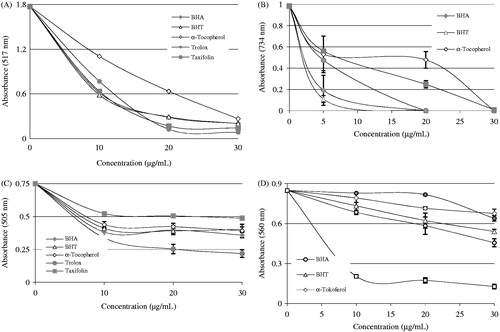
Figure 5. Radical-scavenging activity of taxifolin. (A) DPPH free radical-scavenging activity of different concentrations (10–30 μg/mL) of taxifolin (r2: 0.931) and reference antioxidants. (B) ABTS radical-scavenging activity of different concentrations (10–30 μg/mL) of taxifolin (r2: 0.925) and reference antioxidants. (C) DMPD radical-scavenging activity of different concentrations (10–30 μg/mL) of taxifolin (r2: 0.907) and reference antioxidants. (D) Superoxide anion radical-scavenging activity of different concentrations (10–30 μg/mL) of taxifolin (r2: 0.982) and reference antioxidants (BHA, butylated hydroxyanisole; BHT, butylated hydroxytoluene; DPPH√, 1,1-diphenyl-2-picryl-hydrazyl free radical; ABTS√+, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid; DMPD√+, N,N-dimethyl-p-phenylenediamine radical).