Figures & data
Scheme 1. Chemical synthesis of naproxen (1), ibuprofen (2), and flurbiprofen (3) derivatives possessing a polar pyrrolidinium (13a, 14a, and 15a), 2 -(hydroxymethyl)pyrrolidinium (13 c, 14 c, and 15 c), or a hydroxyethylammonium chloride moiety (13 b, 14 b, and 15 b). Reagents and conditions: (a) oxalyl chloride, DCM, 25° C, 3–12 h; (b) pyrrolidine or l-proline, DCM, 25 °C, 3 h; (c) 2-hydroxyethylamine, DCM, 25 °C, 3 h; (d) LiAlH4, THF, 25 °C (compounds 10a, 10c, 11a, 11c, 12a, and 12c) or reflux (compounds 10b, 11b, and 12b) for 3 h; (e) dry HCl, ether, N2, 25 °C.
Table 1. Cyclooxygenase (COX)-1 and COX-2 inhibition by water soluble NSAID derivatives lacking a carboxylic acid group (13a–13c, 14a–14c, and 15a–15c).
Figure 1. Time-dependent anti-inflammatory effect exerted by naproxen derivatives. (A) Compound 13a, (B) compound 13b, (C) compound 13c; four rats were used in each group (n = 4); individual values represent the average of 40 measurements (10 measurements per animal) ± SEM; (*) = p < 0.05 versus the control (vehicle) group for the time points marked.
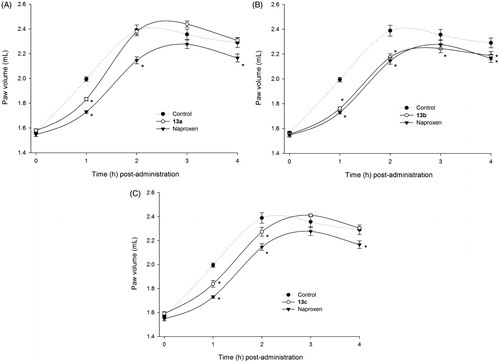
Figure 2. Time-dependent anti-inflammatory effect exerted by ibuprofen derivatives. (A) Compound 14a, (B) compound 14b, (C) compound 14c; four rats were used in each group (n = 4); individual values represent the average of 40 measurements (10 measurements per animal) ± SEM; (*) = p < 0.05 versus the control (vehicle) group for the time points marked.
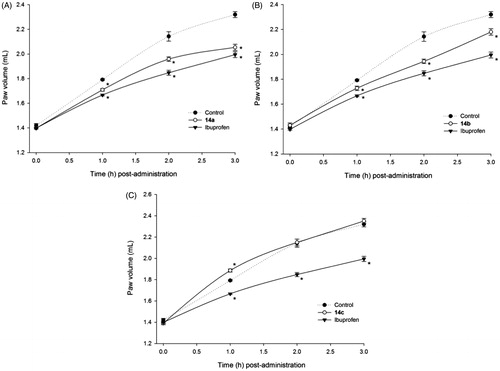
Figure 3. Time-dependent anti-inflammatory effect exerted by flurbiprofen derivatives. (A) Compound 15a, (B) compound 15b, (C) compound 15c; four rats were used in each group (n = 4); individual values represent the average of 40 measurements (10 measurements per animal) ± SEM; (*) = p < 0.05 versus the control (vehicle) group for the time points marked.
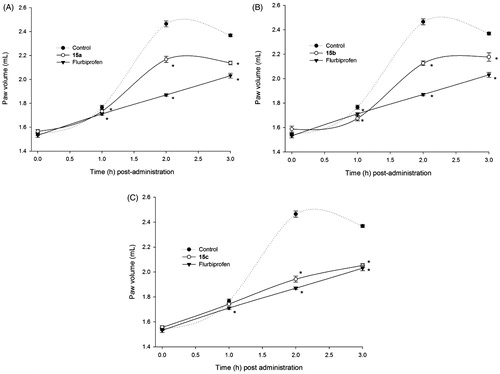
Figure 4. Molecular modeling (docking) of compound 13b (carbon atoms in green color) in the binding site of COX-2 (PDB ID: 6COX; Eintermolecular = −12.23 kcal/mol). Hydrogen atoms of amino acid residues have been omitted for clarity.
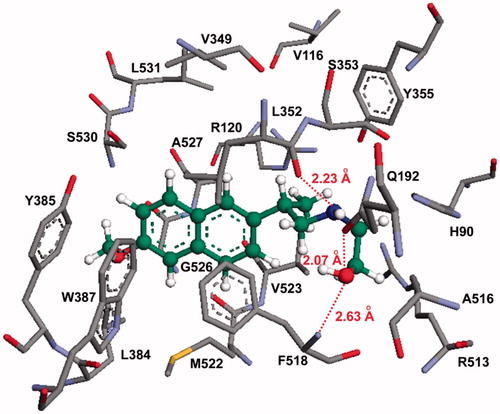
Figure 5. Molecular modeling (docking) of compound 15c (carbon atoms in green color) in the binding site of COX-2 (PDB ID: 6COX; Eintermolecular = −12.16 kcal/mol). Hydrogen atoms of amino acid residues have been omitted for clarity.
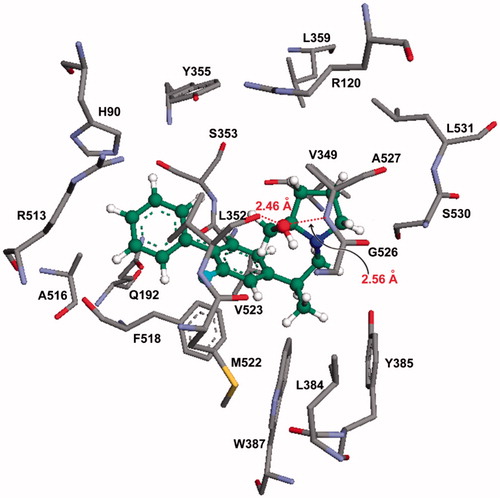
Figure 6. 15-Day anti-inflammatory study comparing flurbiprofen with compound 15a. (A) Percent increase in body weight; (B) percent increase of right paw volumes; (C) percent increase of left paw volumes; (D) percent increase of right joint volumes; (E) percent increase in left joint volume; (F) the arthritic index calculated for compound 15a and flurbiprofen. All values are expressed as percent increase at day 15 compared to day 1; N = 4; doses: flurbiprofen (1 mg/kg orally), 15a (equimolar dose) treated groups. (*) = p < 0.05 versus the control (vehicle) group.
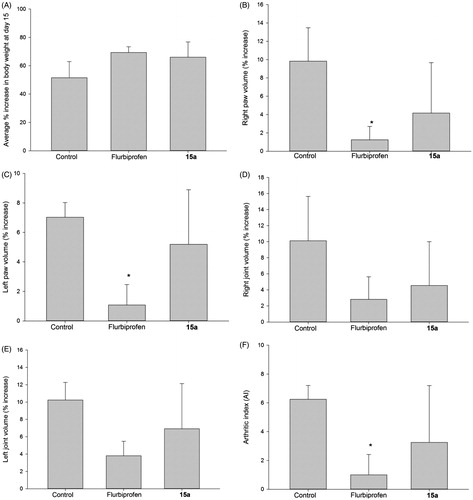
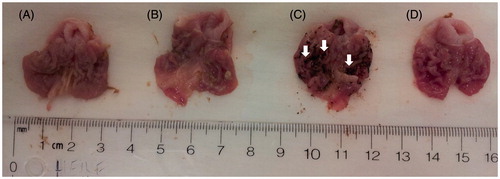
Figure 7. Ulcerogenicity assay data illustrating the extent of NSAID-induced gastric ulcers exerted by a single oral dose of naproxen (40 mg/kg), compared with that observed with derivatives 13a (“A”), and 13c (“B”). Experimental drugs were administered using equimolar doses to that of naproxen (“C”). Control animals (“D”) received methylcellulose as vehicle. Only one (representative) example from each group is presented. White arrows point to areas where we observed significant gastric bleeding.