Figures & data
Figure 1. Clinically used sulfonamide and sulfamate CAIs 1–19 and compound 20 in Phase I clinical development.
Figure 2. CAIs belonging to the zinc binders (sulfonamides, sulfamates, sulfonamides, carboxylates, hydroxamates, phosphonates). The ZBG is coordinated to the metal ion and participates in strong interactions with the gate keeper residues Thr199–Glu106. The scaffold may occupy either the hydrophylic or hydrophobic (or both) halves of the active site, whereas the tails generally are orientated toward the exit of the cavity where the most variable amino acid residues among the different mammalian isoforms are foundCitation1,Citation21,Citation80.
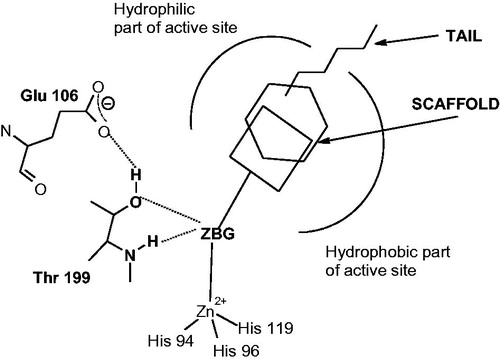
Figure 3. (A) Structure of DTCs 21–23. (B) View of compounds 21 (cyan), 22 (magenta), 23 (green), superposed in the active site of hCA II. The zinc ion is shown as the central blue sphere and the amino acid residues involved in the binding are evidenced and numbered (hCA I numbering system)Citation83.
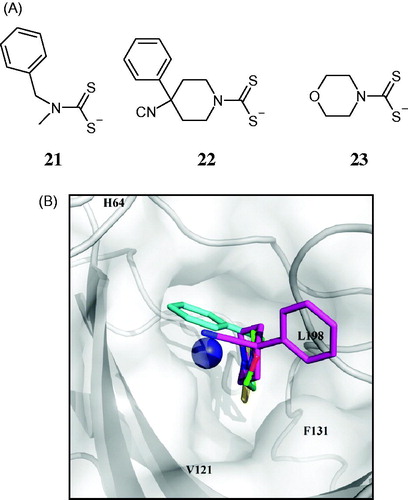
Figure 4. Compounds which anchor to the Zn(II)-coordinated water molecule/hydroxide ion. The anchoring group (AG) may be of the OH (phenol), amino (polyamine), ester (COOR), sulfonate (sulfocoumarin) type or just a simple sulfur atom (thioxocoumarin)Citation103–114.
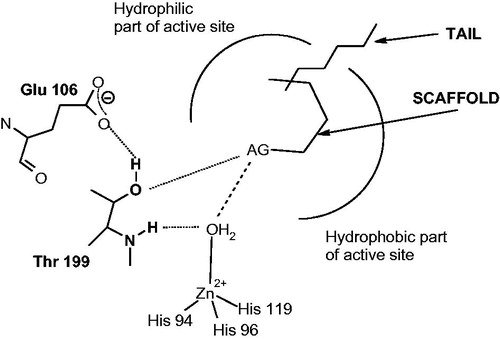
Figure 5. The first compounds which inhibit CAs by anchoring to the zinc-coordinated water molecule: (A) Phenol 24Citation103; (B) Spermine 25Citation104.
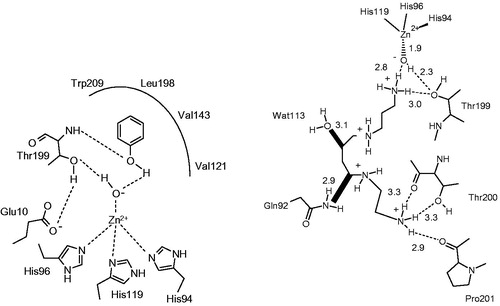
Figure 6. Other compounds which inhibit CAs by anchoring to the non-protein zinc ligand: (A) resorcinol 26Citation113; (B) 2,5-dihydroxybenzoic acid 27Citation113; (C) spermine 25 (stick view, not the schematic one as in Figure 5B)Citation104; (D) xylariamide A 28Citation111; (E) hydrolyzed 6-bromo-sulfocoumarin 29Citation114. (F) Chemical structures of the discussed CAIs. All these adducts have been characterized by high resolution X-ray crystallography. The Zn(II) ion (gray sphere), its three His ligands and coordinated water molecule (red sphere) and amino acid residues involved in the binding of the inhibitors are shown.
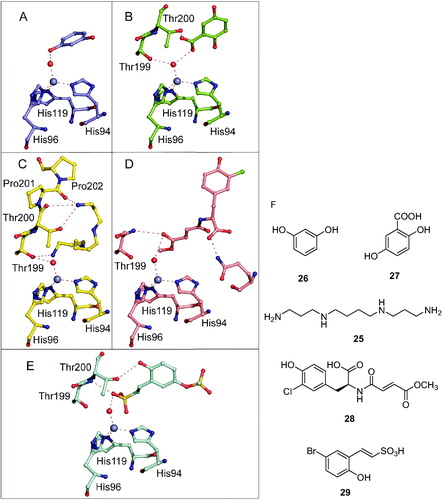
Figure 7. (A) Superposition of the adducts of hCA II with 6-hydroxy-2-thioxocoumarin 30 (sky blue, 4WL4) with the hCA II-hydrolyzed coumarin 31 adduct (5BNL) (silver). The zinc ion, its three His ligands and amino acid residues involved in the binding of inhibitors are shown in Ref. Citation122. (B) Chemical structures of compounds 30 and 31.
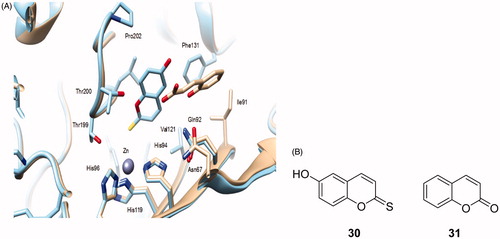
Figure 8. Compounds which inhibit the CAs by occluding the entrance to the active site. SG represents a sticky group, of the phenol, carboxylic acid or amide type, the scaffold binds at the entrance of the active site cavity, occluding it, and a tail may be also present, which will interact with residues on the surface of the proteinCitation115–117.
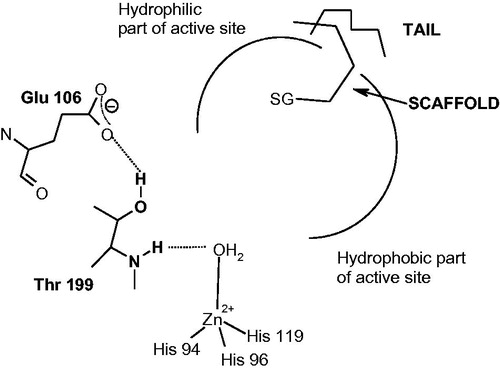
Figure 9. (A) Binding of the coumarin 31 hydrolysis product (trans-2-hydroxy-cinnamic acid 31a, in yellow) and coumarin 32 hydrolysis product (cis-2-hydroxycinnamic acid 32a, magenta) to the hCA II active site. The protein backbone is shown as green ribbon, the catalytic Zn(II) ion as violet sphere, with its three protein ligands (His94, 96, and 119, CPK colors) also evidenced. The proton shuttle residue (His64) is shown in red. (B) Coumarins 31 and 32 and their CA-mediated hydrolysis to 31a and 32a, respectivelyCitation124,Citation125.
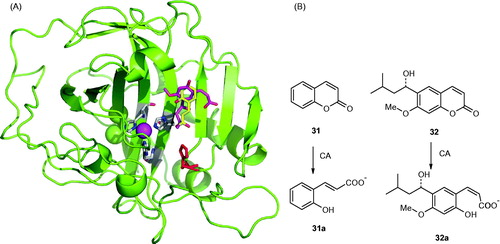
Figure 10. Proposed inhibition mechanism of CAs by coumarins/thiocoumarins, leading to cis- or trans-2-hydroxy/mercapto-cinnamic acids. (A) Hydrolysis of the lactone ring. (B) Movement of the hydrolysis product (as cis stereoisomer) toward the entrance of the active site cavity. (C) Cis-trans isomerization of the hydrolysis productCitation124,125.
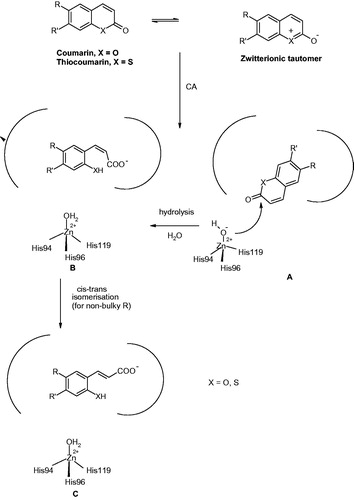
Figure 11. Some of the investigated coumarins 33–51 as CAIs which showed highly isoform-selective inhibition profile against various human CA isoformsCitation124,125.
Figure 12. Out of the active site CA inhibition mechanism. (A) Schematic representation of the inhibitor binding site. (B) hCA II complexed with the carboxylic acid 52. (C) Interactions between the inhibitor 52 and amino acid residues from the binding pocket. (D) Chemical structure of 52Citation139.
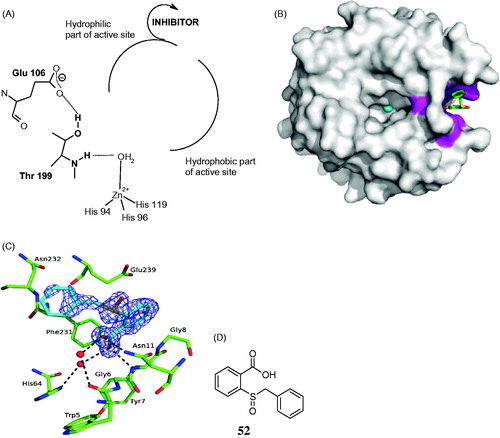
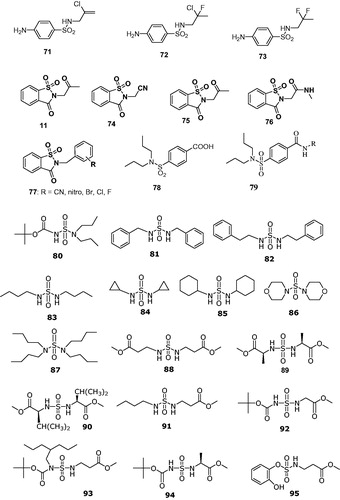
Figure 13. Secondary/tertiary sulfonamides 53–70 initially investigated as CAIsCitation141.
Figure 14. Secondary/tertiary sulfonamides, sulfamates, and sulfamides investigated as CAIsCitation145–154.