Figures & data
Figure 1. Schematic representation of the high hydrostatic pressure (HHP) equipment. The HHP equipment consists of the following components: (1) flanged closure; (2) pressure autoclave; (3) hand pump (SITEC-Sieber Engineering, Maur, Switzerland); (4) reservoir filled with pressure-transmitting media (PTM) (Tivela S220; Shell, Hamburg, Germany); (5) valves SITEC-Sieber Engineering, Maur, Switzerland); and (6) digital pressure sensor. The wrapped sample has to be positioned in the pressure autoclave (2). The pressure autoclave (2) was filled completely with PTM from the reservoir (4; with avoidance of trapped air) and subsequently locked by screwing the flange (1). Pressure was applied manually by means of the hand pump (3) with an approximate pressure increment of 10 MPa/sec. When the aspired pressure was reached, the valve (5) for the pressure autoclave was locked and pressure was held for 300 sec. The pressure was monitored continually via the digital pressure sensor (6). Afterwards, the valve was opened slowly and pressure was decompressed to atmospheric pressure by inverse moving of hand pump (3).
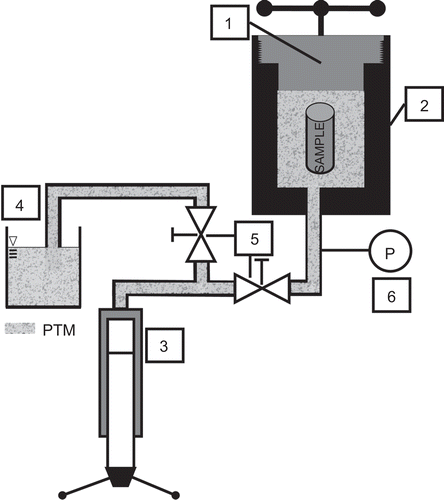
Figure 2. High hydrostatic pressure (HHP) treatment effects on the viability of tumor cells. Viability of the cells was determined by their FSc/SSc properties. The line plots display the percentage of viable MCF7, B16-F10, or CT26 cells after pressure application up to 500 MPa in dependence on culture time. Data (expressed as mean ± SD) were obtained from at least three independent experiments, each performed in duplicate.
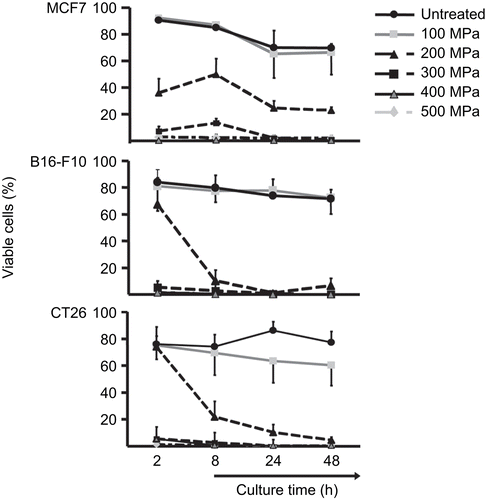
Figure 3. High hydrostatic pressure (HHP) treatment effects upon the levels of necrosis in tumor cells. The bar charts represent the percentage of viable (AxV−/PI−), apoptotic (AxV+/PI−), and necrotic (AxV+/PI+) (A) MCF7, (B) B16-F10, and (C) CT26 tumor cells that were treated with the indicated pressures, as a function of the length of post-treatment culture time. Data (expressed as mean ± SD) were obtained from at least three independent experiments, each performed in duplicate.
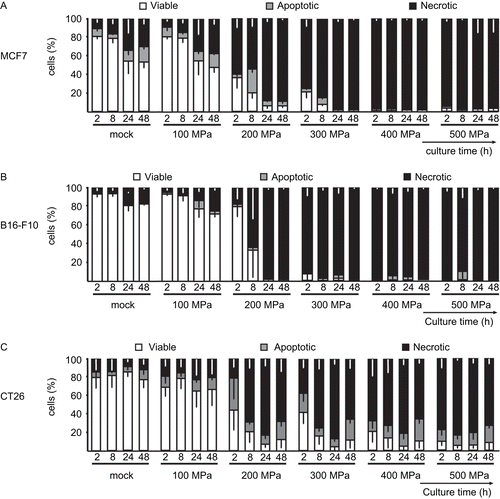
Figure 4. High hydrostatic pressure (HHP) treatment effects upon necrotic tumor cell and degraded DNA content. The DNA content of (A) MCF7, (B) B16-F10, and (C) CT26 cells is illustrated by the bars. The cells were treated with the indicated pressure and then cultured for up to 48 h. The percentages of fractions of cells with sub-G1, G1, and S/G2 content are displayed. Data (expressed as mean ± SD) were obtained from at least three independent experiments, each performed in duplicate.
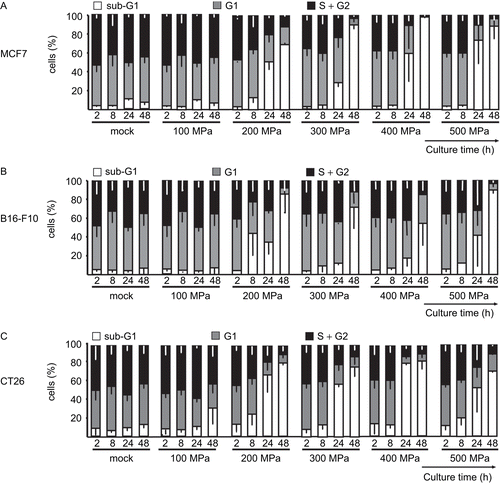
Figure 5. Colony-forming ability of tumor cells after high hydrostatic pressure (HHP) treatment. Line plot represents the percentage of colony-forming units (CFU) [normalized to the CFU associated with untreated (mock) cells] of MCF7, B16-F10, and CT26 cells after HHP treatment. Representative data (expressed as mean ± SD) from independent experiments—each performed in triplicate—are displayed.
![Figure 5. Colony-forming ability of tumor cells after high hydrostatic pressure (HHP) treatment. Line plot represents the percentage of colony-forming units (CFU) [normalized to the CFU associated with untreated (mock) cells] of MCF7, B16-F10, and CT26 cells after HHP treatment. Representative data (expressed as mean ± SD) from independent experiments—each performed in triplicate—are displayed.](/cms/asset/df907107-bfbc-43be-bfe3-7848a34df8bf/iimt_a_466250_f0005_b.gif)
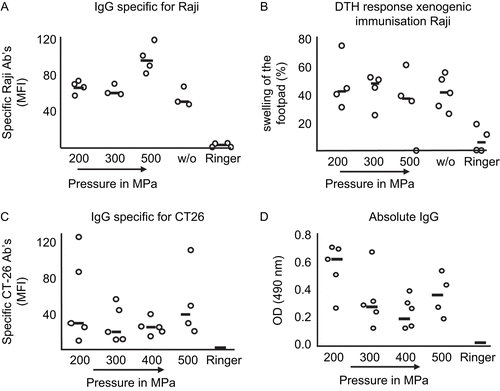
Figure 6. Effects on immunogenicity of xenogeneic and syngeneic high hydrostatic pressure (HHP)-treated tumor cells. Specific IgG antibodies against (A) xenogeneic and (C) syngeneic tumor cells in sera of immunized mice were detected using flow cytometry. (A) C57BL/6 mice were immunized with viable or HHP-treated Raji cells, and (C) Balb/c mice with syngeneic HHP-treated CT26 tumor cells. Serum derived from mice that were injected with Ringer solution served as the control. (B) Delayed-type hypersensitivity test (DTH) response (evaluated by measuring swelling of mice footpads) after injection of xenogeneic tumor cells. (D) Absolute IgG in serum after immunization of Balb/c mice with syngeneic CT26 tumor cells inactivated with the indicated pressure. Each open circle implies a single animal, while the black line is the median of all animals per group. w/o: untreated, viable cells; MFI: mean fluorescence intensity.