Figures & data
Fig. 1. Biogenesis and release of extracellular vesicles.
Extracellular vesicles can be broadly classified into 3 main classes: (a) Microvesicles/microparticles/ectosomes that are produced by outward budding and fission of the plasma membrane; (b) Exosomes that are formed within the endosomal network and released upon fusion of multi-vesicular bodies with the plasma membrane; and (c) Apoptotic bodies are released as blebs of cells undergoing apoptosis. Lower organisms, such as bacteria and parasites, are also able to secrete EVs. Outer membrane vesicles (OVM) are formed by outward bulging of the outer membrane of gram-negative bacteria. EE=early endosome; MVB=multi-vesicular body; ILV=intraluminal vesicles; N=Nucleus; OM=outer membrane; Pp=periplasm; IM=inner membrane; n=nucleoid; F=flagella.
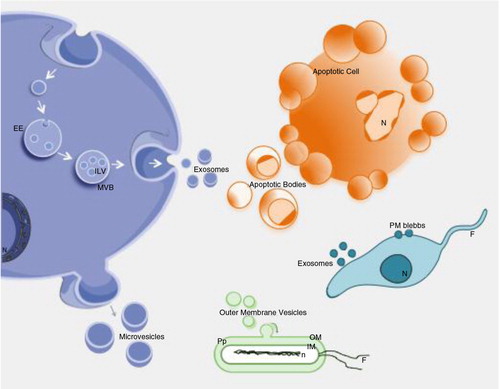
Fig. 2. Curvature sorting mechanism.
In the process of budding, membrane constituents redistribute to regions with fitting membrane curvature to minimize membrane free energy. Redistribution of membrane constituents is then reflected in the pinched off vesicles. As examples, this scheme indicates tetraspanins, ESCRT (Endosomal Sorting Complex Required for Transport) complexes, anonymous integral membrane proteins of a given type, glycoproteins and proteins that are preferentially located in the cell interior and exterior. ESCRT complex favours the neck region of the bud and is disintegrated after the vesicle pinches off. The content that is enclosed by the vesicle membrane becomes mobile and may reach distant cells.
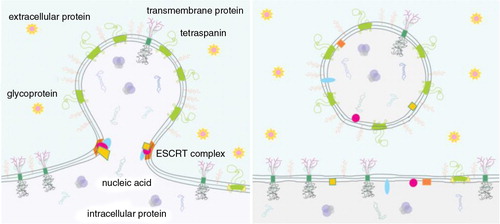
Table I. Examples of EV-associated cytokines
Table II. Lipidomic studies on EVs
Fig. 3. Schematic of in vivo-derived EVs isolated from body fluids.
Cells from different human tissues of the body communicate through the secretion of EVs into proximal body fluids. EVs contain proteins, lipids and RNA molecules that may affect the physiology of cells bathed in or lining these body fluids. Highlighted here are the body fluids where EVs have been identified and their possible cellular origin. Pink spots represent body fluids, which are only present in females. Green spots represent body fluids, which are only present in male. Yellow spots represent body fluids present in both female and male. CSF=cerebrospinal fluid; BALF=bronchoalveolar lavage fluid.
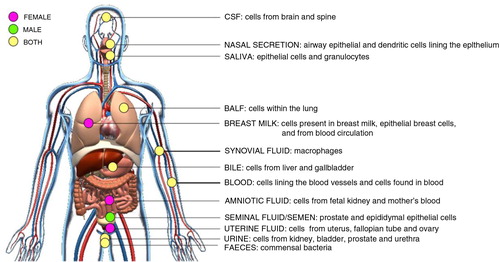
Fig. 4. EVs in coagulation.
Haemostasis: Originating from various sources (monocytes, endothelial cells, platelets), procoagulant (tissue factor (TF)-EVs and phosphatidylserine (PS)-bearing EVs) and anticoagulant, as well as pro-fibrinolytic EVs may circulate at low levels in normal, healthy blood, contributing to the maintenance of the homeostatic balance in blood coagulation. Up-regulated coagulation or thrombosis: Various clinical conditions (cancer, cardiovascular diseases, inflammation, diabetes, sepsis and others) may trigger the coagulation system, activating circulating monocytes and platelets, making endothelial cells procoagulant and resulting in increased generation of procoagulant EVs, particularly TF–EVs, thus leading to a hypercoagulable condition with thrombotic events, hallmarked with fibrin formation and platelet entrapment (thrombus formation).
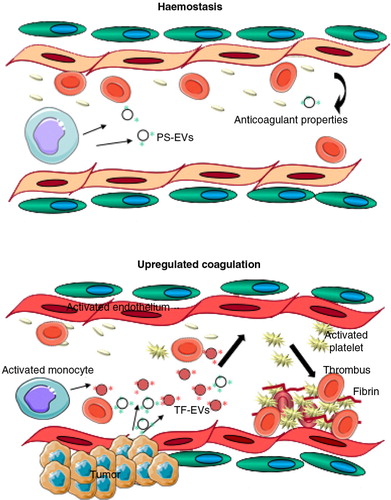
Fig. 5. Physiological role of EVs related to cells of the innate immune system.
Activated macrophages release EVs that contain cytokines, miR-223 and carry out lateral transfer of receptors influencing myeloid cell proliferation and differentiation. Neutrophilic granulocytes (PMN) produce different types of EVs, depending on the type of stimulus. Neutrophil-derived EVs counteract the activation of immune cells or inhibit bacterial growth directly. EVs containing HSP-70 activate NK cells to combat tumour cells. DC=dendritic cell; NK=natural killer; NKG2D=natural killer group 2D; HSP=heat shock protein.
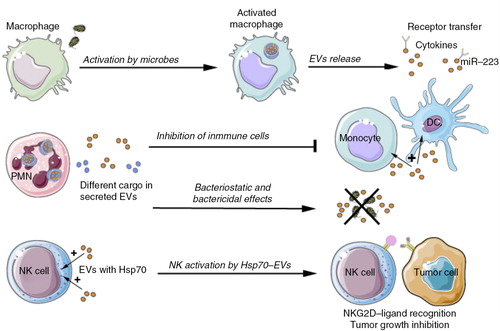
Fig. 6. EVs in the immune system: antigen presentation and acquired immunity.
EVs may have a role in both the origin and progress of the acquired immune response, acting at different levels and on different cells. This figure summarizes how EVs are involved in this process. APC=antigen-presenting cell; Treg=regulatory T cell; NK=natural killer; MHC=major histocompatibility complex.
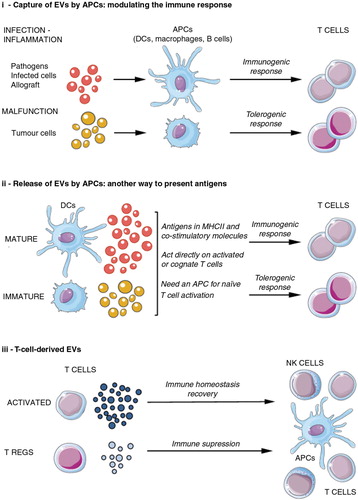
Fig. 7. EVs from mesenchymal stem cells (MSC).
EVs derived from MSCs can induce different effects depending on the target cell, as summarized here. DC=dendritic cell; NK= natural killer.
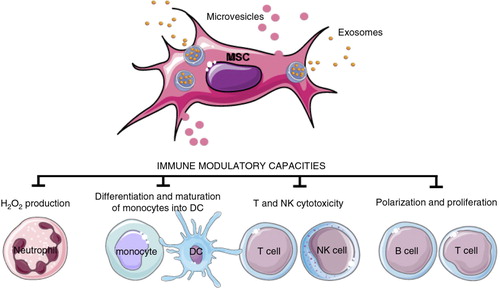
Fig. 8. Summary of functions of EVs released by syncytiotrophoblasts.
Syncytiotrophoblasts and EVs secreted by them (STMBs) control both maternal adaptive and innate immunity during pregnancy, mainly via their non-classical MHC I molecules (HLA–E, HLA–F, HLA–G) expression. STMB play a key role in the induction of local immune tolerance. Cell surface or STMB-bound non-classical MHC–I molecules inhibit the killer activity of NK cells through inhibitory receptors, suppress CTL activity and regulate the local CD4+ Treg cell differentiation. STMB can also act on innate immunity via the modification of neutrophil function and NET formation. uNK=uterine natural killer cells; Tc=cytotoxic T cell; Tact=activated T cells; Treg=regulatory T cell; Th=T helper cell; PMN=polymorphonuclear granulocyte, neutrophil.
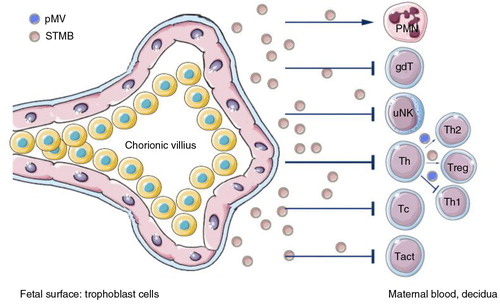
Fig. 9. EVs in the male reproductive tract and seminal plasma.
The epithelial cells of the epididymis produce a population of EVs that are thought to bud directly from the plasma membrane. These vesicles, called epididymosomes, fuse with sperm cells to transfer proteins that contribute to the maturation of sperm cells. Epithelial cells of the prostate also secrete EVs. These vesicles, sometimes termed prostasomes, have been suggested to originate largely from MVB. Prostasomes are thought to interact with sperm cells in the female reproductive tract to facilitate them reaching the oocyte.
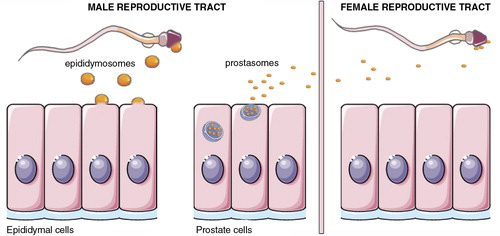
Fig. 10. EVs in parasitic diseases.
Secretion of EVs has been described for both helminths and parasitic protozoa. In helminths, they serve as mechanism for protein and miRNA export and host manipulation. In parasitic protozoa from the kinetoplastids family, EVs released by Leishmania spp. are able to induce specific recruitment of neutrophils to the site of infection. They are also taken up by phagocytic cells, enabling the delivery of immunomodulatory proteins contributing to the creation of a permissive environment for the infection. In T. cruzi, EVs contribute to the stabilization of the C3 convertase disturbing the functioning of the complement system. Regarding Apicomplexa in malaria, circulating levels of EVs rise during human infections and in rodent models, while exosomes derived from reticulocytes induced protection upon immunization in a murine model. Also, exosomes from malarial infections were able to induce parasite sexual development. Other obligate intracellular parasitic protozoa are Toxoplasma gondii and Trichomonas vaginalis. EVs isolated from dendritic cells and primed with Toxoplasma antigens conferred protection upon immunizations being a proof-of-concept of EVs as therapeutics agents. In trichomoniasis EVs increased virulence by inducing parasite attachment to cervical epithelium, thus facilitating host cell colonization.
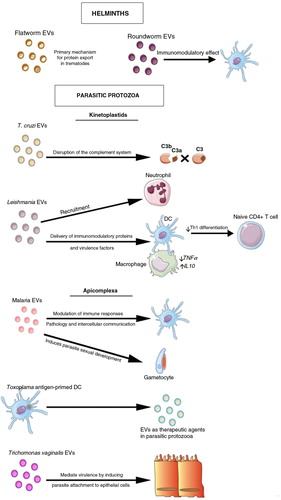
Fig. 11. Biogenesis of an outer membrane vesicle (OMV).
Initialized by outward bulging of the outer membrane, vesicles bud off the gram-negative cell and are then termed OMVs. The membrane of the vesicle is derived from the outer membrane and the lumen contains proteins and other biomolecules from the periplasma.
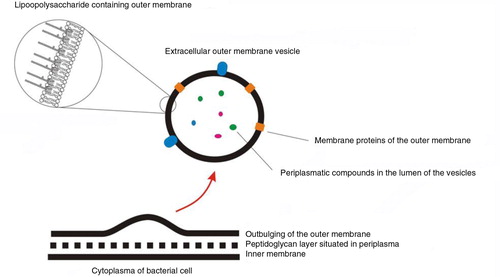
Table III. A summary of 14 papers selected from 180 Pubmed papers published between 1991 and 2013, on the subject of “outer membrane vesicles (OMVs),” published in international journals that studied, using either in vivo or in vitro models, the direct effects on OMVs on human cells
Fig. 12. Putative role of EVs in the pathogen attack response in plants.
Schematic representation of a plant cell penetrated by hyphae from powdery mildew fungus. Pathogen attack results in transport of defence compounds to the attack location near the plasma membrane by polarization of the cytoskeleton. Two routes of vesicle secretion have been identified: (a) Golgi-derived vesicle secretion relying on SNARE complex formation at the plasma membrane and (b) Fusion of MVBs with the plasma membrane leading to release of intraluminal vesicles (exosomes) into the paramural space (in between the cell wall and plasma membrane). Cross-talk may exist between MVBs and the trans Golgi network/early endosomes in sorting proteins for MVB-mediated degradation or recycling/exosome secretion to the plasma membrane. Plasmodesmata connect the paramural space across cell walls and may facilitate transport of cargo released from vesicles over longer distances. [Figure adapted from (Citation714, Citation715)].
![Fig. 12. Putative role of EVs in the pathogen attack response in plants.Schematic representation of a plant cell penetrated by hyphae from powdery mildew fungus. Pathogen attack results in transport of defence compounds to the attack location near the plasma membrane by polarization of the cytoskeleton. Two routes of vesicle secretion have been identified: (a) Golgi-derived vesicle secretion relying on SNARE complex formation at the plasma membrane and (b) Fusion of MVBs with the plasma membrane leading to release of intraluminal vesicles (exosomes) into the paramural space (in between the cell wall and plasma membrane). Cross-talk may exist between MVBs and the trans Golgi network/early endosomes in sorting proteins for MVB-mediated degradation or recycling/exosome secretion to the plasma membrane. Plasmodesmata connect the paramural space across cell walls and may facilitate transport of cargo released from vesicles over longer distances. [Figure adapted from (Citation714, Citation715)].](/cms/asset/6a9113b0-79ec-4013-b61e-9bdf8f24049a/zjev_a_11815543_f0012_ob.jpg)

