Figures & data
Figure 1. Base Peak Electropherogram corresponding to the analysis by CESI-MS/MS of trastuzumab tryptic digest. Experimental conditions: bare fused silica capillary with porous tip, total length 95 cm (30 μm i.d., 150 μm o.d.); CE voltage +20 kV; BGE 10% acetic acid; sample trastuzumab tryptic digest 2.5 mg/mL in BGE (11nL injected). MS capillary voltage: -1.3 kV, m/z range: 50–3000.
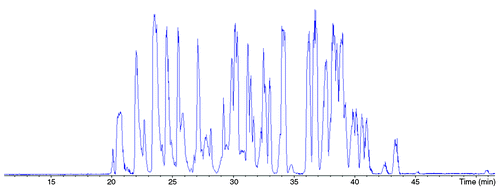
Figure 2. Sequence coverage obtained by CESI-MS/MS for trastuzumab HC (left-hand side) and LC (right-hand side) based on the identified peptides. Experimental conditions: see materials and methods

Figure 3. List of trastuzumab digested peptides identified by the CESI-MS/MS analysis. Experimental conditions: see material and methods

Figure 4. MS/MS spectra of peptide HT01 and HT02 illustrating the amino acid sequence retracing in the variable domain. Experimental conditions: see material and methods.
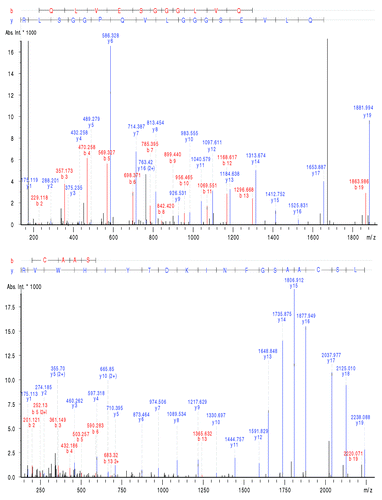
Figure 5. MS/MS spectra of peptide HT04 (IYPTNGYTR) (A) without modification and (B) with a deamidation on Asn55 illustrating the partial modification of the mAb and the characterization of both forms by CESI-MS/MS. Experimental conditions: see Materials and Methods.
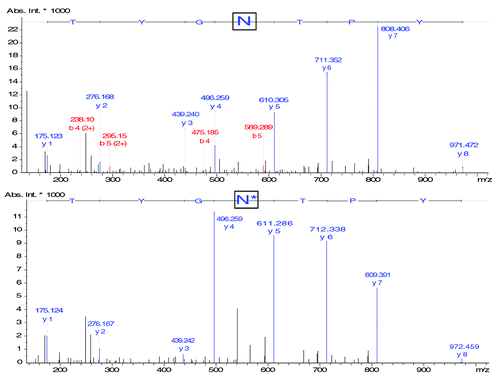
Figure 6. Extracted Ion Electropherogram of m/z ratios (A) 1039.80 (HT29 + G0F), (B) 1093.78 (HT29 + G1F), (C) 972.09 (HT29 + G0), (D) 1147.82 (HT29 + G2F), (E) 991.10 (HT29 + G0). Experimental conditions: see Material and Methods.
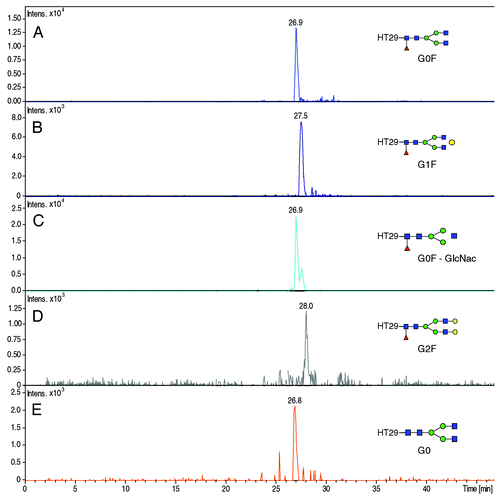
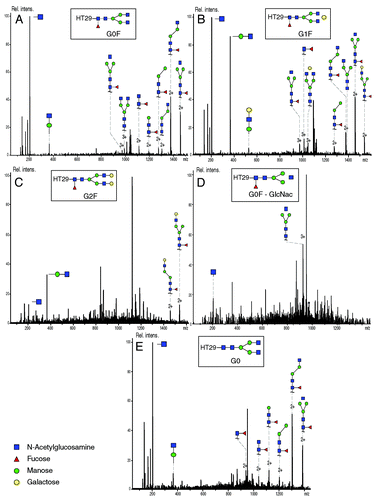
Figure 7. MS/MS fragmentation spectra of (A) HT29 - G0F (precursor m/z 1039.78; charge state 3+), (B) HT29 - G1F (precursor ion 1093.80; charge state 3+), (C) HT29 - G2F (precursor ion 1147.82; charge state 3+), (D) HT29 - G0F-GlcNac (precursor ion 972.10; 3+), (E) HT29 - G0 (precursor ion 991.09; charge state 3+). Experimental conditions described in Materials and Methods section.