Figures & data
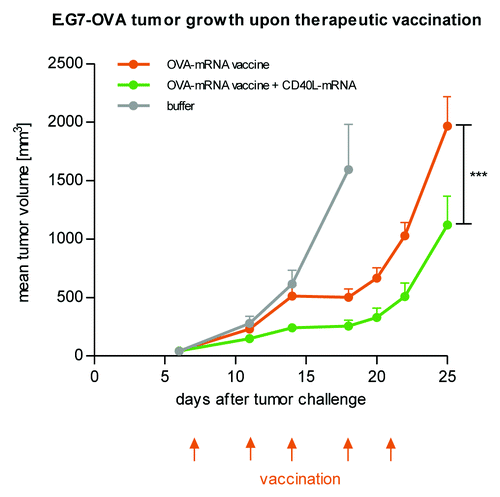
Figure 1. Protein expression in vivo is strongly prolonged using CureVac’s proprietary mRNA technology and lasts for many days. Firefly luciferase-encoding mRNA, optimized for translation and stability, was injected intradermally in a BALB/c mouse (4 injection sites). At various time points after mRNA injection, luciferase expression was visualized in the living animal by optical imaging. (A) Visualization of luciferase expression at selected time points, showing maximal protein levels 24 to 48 h after mRNA injection. (B) Time course of luciferase expression until 9 d after mRNA injection. Background signal was set to 1.
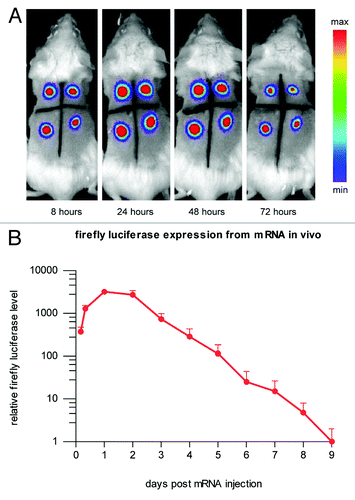
Figure 2. A biologically relevant increase of reticulocytes is induced in mice using CureVac’s proprietary mRNA technology. A single intramuscular injection in BALB/c mice of erythropoietin (Epo)-encoding mRNA, optimized for translation and stability, causes the expression of functional Epo. Reticulocyte levels are raised comparably by mRNA and recombinant protein injected intramuscularly.
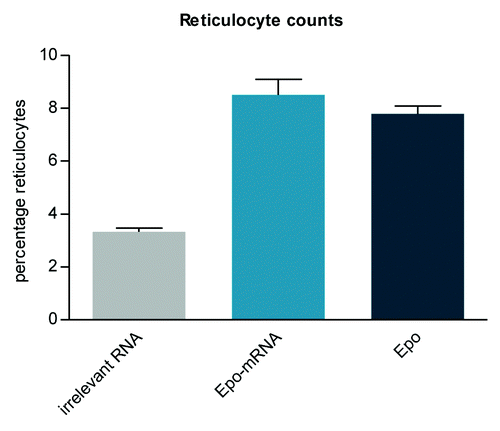
Figure 3. CD40 ligand as an accessory adjuvant molecule encoded by mRNA increases the anti-tumor effect of a two-component mRNA vaccine. Mice (n = 8 per group) were challenged subcutaneously with syngenic E.G7-OVA tumor cells on day 0. Commencing on day 7, mice were vaccinated intradermally with either OVA-mRNA vaccine alone or in combination with mRNA coding for CD40 ligand according to the indicated schedule. Mice treated with buffer served as the control. The combination of CD40 ligand-encoding mRNA together with OVA mRNA vaccination increases the efficacy of the therapeutic anti-tumor mRNA vaccination.